Invasion History
First Non-native North American Tidal Record: 1978First Non-native West Coast Tidal Record: 1978
First Non-native East/Gulf Coast Tidal Record: 1996
General Invasion History:
Caprella scaura was first described from Mauritius in 1836, and subsequently found in tropical and subtropical areas around the world. Historically, it ranged as far north as Vladivostok, Russia, in the Northwest Pacific, and south to Chile, South Africa, and southern Australia (McCain 1968). Its native region is uncertain. In other parts of the world, it has recently extended its range into warm-temperate regions, including California (Marelli 1981), South Carolina, the Gulf Coast of Florida (Foster et al. 2004), the Atlantic coast of Spain, and the Canary Islands (Guerra-Garcia et al. 2011). In the Mediterranean, there was an early report from the Thau Lagoon, France, an oyster-rearing area (Razarihelisoa 1958), but it has appeared in other regions of the Sea more recently (Krapp et al. 2006; Martínez and Adarraga 2008; Zenetos et al. 2008; Bakir and Katagan 2011). The morphological variability of this caprellid indicates that the name C. scaura is a species complex, but many of the introduced populations are morphologically similar and appear to correspond to Mayer's (1890, cited by McCain 1968) form ‘typica’. Caprella scaura is capable of long-distance dispersal on floating seaweeds and other objects. However, its worldwide distribution has probably resulted from transport by hull fouling, ballast water, and by oyster transplants and other forms of aquaculture (Foster et al. 2004; Krapp et al. 2006; Martínez and Adarraga 2008; Guerra-Garcia et al. 2011).
North American Invasion History:
Invasion History on the West Coast:
Caprella scaura was first collected on the West Coast in 1978 by Marelli (1981) from the cooling systems of power plants in San Francisco Bay and Elkhorn Slough, California. It was subsequently collected in Southern California, from the Channel Islands, Oxnard, and San Diego Bay (Fairey et al. 2002).
Invasion History on the East Coast:
Caprella scaura was collected during benthic surveys on the East Coast of Florida, in Biscayne Bay in 1996, and the St. Johns River estuary in 2001 (NOAA National Benthic Inventory 2012). In 2002, it was found in the fouling community of a floating dock in Charleston Harbor, South Carolina (Foster et al. 2004).
Invasion History on the Gulf Coast:
In 1998-2002, Caprella scaura was found near Panama City, Florida in St. Andrews Bay on jetties and channel markers (Foster et al. 2004).
Invasion History in Hawaii:
Caprella scaura was first found in Pearl Harbor, Oahu in 1929 (Coles et al. 1999a; Calton and Eldredge 2009). Subsequently, it was found in other Oahu harbors (Coles et al. 1999b), and in 2003, in Malea Harbor, Maui (Coles et al. 2004).
Invasion History Elsewhere in the World:
As noted above, the type locality of C. scaura is Mauritius. Mayer (1890, cited by Krapp et al. 2006) obtained type specimens for the subspecies typica and cornuta in Rio de Janeiro, while the type locality for his diceros and Utinomi's (1947, cited by Krapp et al. 2006) hamata form was Japan. These forms all lack a ventral spine between gnathopods 2, and may be conspecific or closely related (Krapp et al. 2006). In any event, C. scaura s. l. was distributed in the South Atlantic, Pacific and Indian Oceans by the late 19th century. By 1968, C. scaura was known from the Caribbean, the Southwest Atlantic (Brazil), the Southeast Pacific (Chile), the Southwest Pacific (Sydney, Australia), the Northwest Pacific (Vladivostok to southern Japan), and the Southeast Atlantic (South Africa) (McCain 1968). More recent records include New Caledonia (Laubitz 1991), St. Paul's Island (Southern Indian Ocean, Laubitz 1995), and Hong Kong (Guerra-Garcia and Takeuchi 2003). We consider most of this range cryptogenic, given the potential for natural dispersal, and early, undocumented transport of this caprellid by ships.
Recent expansions of C. scaura in several parts of the world appear to be introductions. In Australia, the caprellid was found in Port Philip Bay, Victoria in 1990 (Museum Victoria 2012), in Tasmania in 1980 (Guerra-Garcia and Takeuchi 2004), and in Western Australia in 1983 (Guerra-Garcia 2004). In Europe, there was an early report of C. scaura in the Mediterranean, in the Thau Lagoon, a major oyster-rearing area (Razarihelisoa 1958). However, its range in the Mediterranean and Eastern Atlantic has expanded greatly in the last two decades. Caprella scaura was seen in the Venice Lagoon in 1994 (Krapp et al. 2006), and subsequently appeared in the Ionian Sea, Greece (in 2002, Krapp et al. 2006), the Aegean Sea, Turkey (in 2008, Bakir and Katagan 2011), and Western Mediterranean locations in Spain and Italy (Martínez and Adarraga 2008). In the Eastern Atlantic, this caprellid appeared off Cadiz, Spain and in great abundance in aquaculture operations in the Canary Islands, in 2009 (Guerra-Garcia et al. 2011).
Description
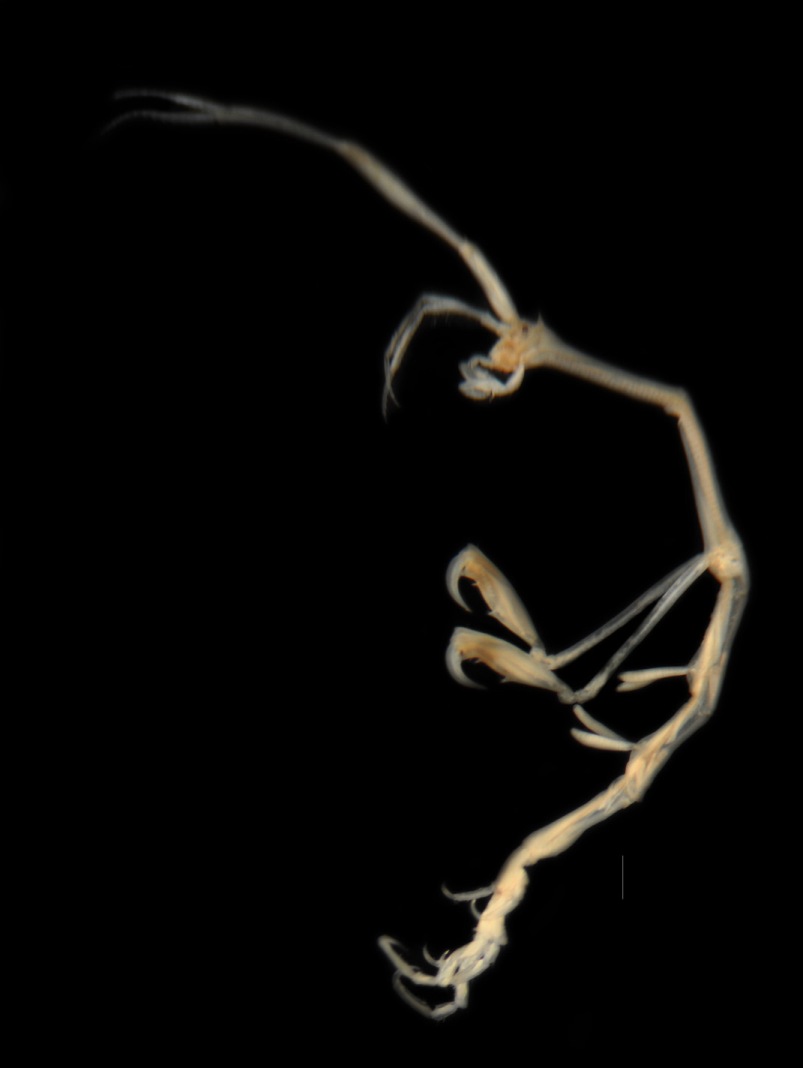
Caprellid amphipods have a greatly modified body form, when compared to more familiar gammarid amphipods. The body is elongated (giving rise to the name 'skeleton shrimp'), though the abdomen is compressed. The head is partly fused with the first thoracic segment (called Pereonite 1 in amphipods). The head bears a pair of long antennae 1, somewhat shorter antennae. The 1st antennae (A1) have a 3-segmented peduncle, tipped by a flagellum with multiple segments. The 2nd antennae (A2) may be fringed with long setae, and have 3-4 segments in the peduncle, and a shorter flagellum, usually of 2 segments. A mandibular palp of several segments is present in some genera, arising between the antennae, but this is absent in Caprella. There is a small pair of gnathopods, small grasping claws with a movable finger (Gnathopod 1), on Pereonite 1. Pereonite 2 bears a much larger pair of gnathopods (Gnathopod 2), which may have conspicuous spines or setae. Pereonites 3 and 4 usually have round or club-shaped gills, while in most species, including Caprella, pereiopods are absent. Pereiopods 5, 6, and 7 are roughly equal and hook-like, for climbing and attachment, with 6 segments. Females develop oostegites, plates which form a brood pouch. Males are usually larger than females of the same species. Females and immature males can be hard to identify to species level. (Description from: Barnes 1983; Watling and Carlton, in Carlton 2007).
Caprella scaura are distinguished by a sharply pointed, forward-pointing dorsal spine on the head. Mature male gnathopod 2 propodus may be extended distally, with a mid-palm proximal spine, distal poison tooth and triangular projection; dactylus may be sculpted However, the size of specimens can vary considerably by region and season. There are a number of morphological differences between male and females. In females, flagellum of the antennule has up to four segments, usually fewer than the male. The pereonites of females are greatly shortened compared with those of the males. Females vary in the number of body spines, with as many as one or two knobs on each pereonite. The propodus of gnathopod 2 of females is less elongated than males, and has one proximal spine, and a small distal tooth. Mature females are distinguished by the developing oostegites and brood pouch. See descriptions by: McCain 1968; Guerra-Garcia and Takeuchi 2003; Martínez and Adarraga 2008; Guerra-Garcia et al. 2011.
This 'species' shows a high degree of variation in several anatomical features. Mayer (1890, cited by McCain 1968) named six varieties (now subspecies): typica, cornuta, diceros, scauroides, spinirostris, and californica. An additional one, hamata was added by Utinomi (1947, cited by McCain 1968; Krapp et al. 2006). One of the distinguishing features of the subspecies is the absence of a ventral spine between the insertions of Gnathopods 2 in typica, cornuta, and diceros, and its presence in the other named forms, including californica, scauroides, and spinirostris. Caprella californica, from the Northeast Pacific, is now recognized as a full species (McCain 1968; Watling and Carlton, in Carlton 2007). The other ventral-spined forms, scauroides and spinirostris, may be conspecific with C. californica (Laubitz 1970, cited by Guerra-Garcia and Takeuchi 2004). The currently recognized subspecies of Caprella scaura appear to constitute a widespread species complex. Molecular studies will be needed to clarify their systematics and origin. However, specimens caught in US Atlantic and Pacific waters resemble C. s. typica (McCain 1968; Marelli 1981; Foster et al. 2004).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Arthropoda | |
| Subphylum: | Crustacea | |
| Class: | Malacostraca | |
| Subclass: | Eumalacostraca | |
| Superorder: | Peracarida | |
| Order: | Amphipoda | |
| Suborder: | Caprellidea | |
| Infraorder: | Caprellida | |
| Superfamily: | Caprelloidea | |
| Family: | Caprellidae | |
| Genus: | Caprella | |
| Species: | scaura |
Synonyms
Caprella attenuata forma subtenuis (Dana, 1853)
Caprella cornuta (Dana, 1853)
Caprella cornuta forma obtusirostris (Dana, 1853)
Caprella nodosa (Templeton, 1836)
Caprella scaura forma cornuta (Mayer, 1890)
Caprella scaura forma diceros (Mayer, 1890)
Caprella scaura forma hamata (Mayer, 1890)
Caprella scaura forma typica (Mayer, 1890)
Potentially Misidentified Species
NE Pacific native, formerly considered a subspecies of C. scaura (Cabezas et al. 2014)
Caprella penantis
Cosmopolitan, a species complex
Caprella scauroides
NW Pacific native, Taiwan
Caprella simia
NW Pacific native, introduced to California
Ecology
General:
Life History – Males and females are morphologically distinct. The males are larger, more robust, and armed with larger gnathopods, probably an adaptation to competition for females and for guarding themselves during molting, which precedes mating. The young are brooded by the female in an egg-pouch formed by large plates (oostegites) on the 3rd and 4th pereionites (Turcotte and Sainte Marie 2009). Brooding takes about four days at 24⁰C (Lim and Alexander 1986). Development is direct, with the newborn juveniles having the general form of the adults. The 1st instar of Caprella scaura are quite small (~1.2 mm), lacking the distinctive head spine, and having less developed gnathopods, however perieopods with long dactyls, specialized for clinging to the mother are observed even in small individuals (Lim and Alexander 1986). The young are dependent on maternal care and cling to females for about a week after birth. Brooding females and females with newborns are more likely to fight with males than are non-reproducing females (Lim and Alexander 1986; Aoki 1999).
Ecology – Caprellids can feed in a variety of ways, including filtering small particles from the water, browsing on small filamentous algae, scraping tissue from large algae, scavenging, and predation (Turcotte and Sainte Marie 2009). An examination of the gut contents (specimens from Mauritius, Australia, Greece, and Chile) found that 99.8% of their diet was detritus (Guerra-Garciana and de Figeroa 2009). A later study found a dietary shift during development, with juvrniles doing more predatory feeding, and then shifting to detritus feeding as adults (Ros et al. 2014). Caprella scaura is known for using a wide range of habitats, including seaweeds, seagrasses, sponges, hydroids, bryozoans (Zoobotryon verticillatum, Bugula neritina), and manmade structures such as buoys, floating docks, and aquaculture systems (McCain 1968; Thiel et al. 2003; Foster et al. 2004; Guerra-Garcia et al. 2011; Ros et al. 2013; Ros et al. 2016; Molina et al. 2017). In short term experiments, C. scaura tolerated salinities as low as 7.5 PSU (Cockman and Albone 1987, cited by Ros et al. 2015), but on the Mediterranean and Atlantic coasts of Spain,C. scaura predominated over C. equilibria at salinities of 36-38 PSU (Ros et al. 2015).
Food:
Phytoplankton, detritus
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Grass Bed | None |
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Oyster Reef | None |
| General Habitat | Marinas & Docks | None |
| General Habitat | Rocky | None |
| General Habitat | Unstructured Bottom | None |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 7 | Field, Mar Piccolo, Italy, Mediterranean Sea (Prato et al. 2013) |
| Maximum Temperature (ºC) | 30 | Field, Mar Piccolo, Italy, Mediterranean Sea (Prato et al. 2013) |
| Minimum Salinity (‰) | 7.5 | Experimental, 24 hr LC 50, Swan River, Western Australia, Cockman and Albone 1987, cited by Ros et al. (2015). |
| Maximum Salinity (‰) | 38 | Field, Mar Piccolo, Italy, Mediterranean Sea (Prato et al. 2013) |
| Minimum Length (mm) | 5.6 | For mature breeding females,Mar Piccolo, Italy, Mediterranean Sea, Prato et al. 2013) |
| Maximum Length (mm) | 23 | Mar Piccolo, Italy, Mediterranean Sea (Prato et al. 2013) |
| Broad Temperature Range | None | Warm temperate-Tropical |
| Broad Salinity Range | None | Polyhaline-Euhaline |
General Impacts
Caprella scaura has reached very high densities in some introduced locations, including Cadiz Harbor (Guerra-Garcia et al. 2011) and Roses Bay, Spain (Martinez and Adaragga 2008); the Venice Lagoon (Krapp et al. 2006); and in aquaculture operations in the Canary Islands (Guerra-Garcia et al. 2011). In Cadiz Harbor, C. scaura apparently displaced native caprellids and other amphipods (Guerra-Garcia et al. 2011). While this caprellid has been associated with fish culture, negative impacts on aquaculture have not been reported.Regional Impacts
| NEA-V | None | Ecological Impact | Competition | ||
| In the harbor of Cadiz, Spain, Caprella scaura has apparently replaced native Caprella equilibra or Caprella dilatata and apparently reduced the abundance of other native amphipods (Guerra-García et al. 2011). On the Atlantic and Mediterranean coasts of Spain, C. scaura tended to replace C. equilibria at salinities at 36-38 PSU, and higher temperatures (Ros et al. 2015) | |||||
| MED-I | None | Ecological Impact | Competition | ||
| On the Atlantic and Mediterranean coasts of Spain, C. scaura tended to replace C. equilibria at salinities at 36-38 PSU, and higher temperatures (Ros et al. 2015). | |||||
| MED-II | None | Ecological Impact | Competition | ||
| On the Atlantic and Mediterranean coasts of Spain, C. scaura tended to replace C. equilibria at salinities at 36-38 PSU, and higher temperatures (Ros et al. 2015) | |||||
| WA-I | None | Ecological Impact | Competition | ||
| In experiments in Madeira, Caprella scaura tended to displace the cryptogenic C, equilibia, and this effect was greater at 29 C compared to 29 C, suggesting that C. scaura's abundance will be favored by climate warming. | |||||
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Aasen, Geir; Sweetnam, Dale A.; Lynch. Lisa M. (1998) Establishment of the wakasagi, Hypomesus nipponensis in the Sacramento-San Joaquin estuary., California Fish and Game 84(1): 31-35Amor, Kounofi-Ben; Rifi, M.; Ghanem, R.; Draief, I.; Zouali, J.; Souissi, J. Ben (2016) Update of alien fauna and new records of Tunisian marine fauna, Mediterranean Marine Science 17(1): 124-143
Aoki, Masakazu (1997) Comparative study of mother-young association in caprellid amphipods: Is maternal care effective?, Journal of Crustacean Biology 17(3): 447-458
Aoki, Masakazu (1999) Morphological characteristics of young, maternal care behaviour and microhabitat use by caprellid amphipods, Journal of the Marine Biological Association of the United Kingdom 79: 629-638
Ashton, Gail 2012 LifeDesks- <i>Caprella scaura</i> Templeton, 1836. <missing URL>
Bakir, Kerem; Katagan, Tuncer (2011) On the occurrence of Caprella scaura, Templeton, 1836 (Crustacea: Amphipoda) in Turkish waters, Zoology in the Middle East 52: 125-126
Banks, Stuart; Vera, Mariana; Chiriboga, Angel (2021) Establishing reference points to assess long-term change in zooxanthellate coral communities of the northern Galapagos coral reefs, Galapagos Research 66: 43-64
Barnes, Robert D. (1983) Invertebrate Zoology, Saunders, Philadelphia. Pp. 883
Boltovskoy D, Paolucci E, MacIsaac HJ, Zhan A, Xia Z, Correa N (2022) What we know and don’t know about the invasive golden mussel Limnoperna fortunei, Hydrobiologia 852: 1275–1322
https://doi.org/10.1007/s10750-022-04988-5
Cabezas, M. Pilar; Xavier, Raquel; Branco, Madalena; Santos, Antonio M. Guerra-Garcia, Jose Manuel G (2014) Invasion history of Caprella scaura Templeton, 1836 (Amphipoda: Caprellidae) in the Iberian Peninsula: multiple introductions revealed by mitochondrial sequence data, Biological Invasions 16(10): 2221-2245
California Department of Fish and Wildlife (2014) Introduced Aquatic Species in California Bays and Harbors, 2011 Survey, California Department of Fish and Wildlife, Sacramento CA. Pp. 1-36
Carlton, James T. (Ed.) (2007) The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon Fourth Edition, Completely Revised and Expanded, University of California Press, Berkeley. Pp. <missing location>
Carlton, James T.; Eldredge, Lucius (2009) Marine bioinvasions of Hawaii: The introduced and cryptogenic marine and estuarine animals and plants of the Hawaiian archipelago., Bishop Museum Bulletin in Cultural and Environmental Studies 4: 1-202
Cataldo D, Vinocur A, O′Farrell I, Paolucci E, Leites V, Boltovskoy D (2012) The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande reservoir (Argentina): evidence from mesocosm experiments, Hydrobiologia 680(1): 25–38
https://doi.org/10.1007/s10750-011-0897-8
Chainho, Paula and 20 additional authors (2015) Non-indigenous species in Portuguese coastal areas, lagoons, estuaries, and islands, Estuarine, Coastal and Shelf Science <missing volume>: <missing location>
Child, C. Allan (1979) Shallow-water pycnogonida of the isthmus of Panama and the coasts of middle america, Smithsonian Contributions to Zoology 293: 1-86
Çinar, Melih Ertan and 7 authors (2021) Current status (as of end of 2020) of marine alien species in Turkey, None 16: Published online
Coles S. L., DeFelice R. C., Eldredge, L. G. (1999a) Nonindigenous marine species introductions in the harbors of the south and west shores of Oahu, Hawaii., Bishop Museum Technical Report 15: 1-212
Coles S. L., DeFelice R. C., Eldredge, L. G. (2002b) Nonindigenous marine species at Waikîkî and Hawai`i kai, Oahu, Hawai`i, Bishop Museum Technical Report 25: 1-255
Coles, S. L.; DeFelice, R. C.; Eldredge, L. G.; Carlton, J. T. (1999b) Historical and recent introductions of non-indigenous marine species into Pearl Harbor, Oahu, Hawaiian Islands., Marine Biology 135(1): 147-158
Coles, S. L.; Reath, P. R.; Longenecker, K.; Bolick, Holly; Eldredge, L. G. (2004) <missing title>, Hawai‘i Community Foundation and the U. S. Fish and Wildlife Service, Honolulu. Pp. 1-187
Eleftheriou, A. and 26 Authors (2011) New Mediterranean biodiversity records (December 2011), Mediterranean Marine Science 12(2): 491-508
Fairey, Russell; Dunn, Roslyn; Sigala, Marco; Oliver, John (2002) Introduced aquatic species in California's coastal waters: Final Report, California Department of Fish and Game, Sacramento. Pp. <missing location>
Florida Museum of Natural History 2009-2013 Invertebrate Zoology Master Database. <missing URL>
Foster, John M.; Heard, Richard W.; Knott, David M. (2004) Northern range extensions for Caprella scaura (Crustacea: Amphipoda: Caprellidae) on the Florida Gulf Coast and in South Carolina., Gulf Research Reports 16: 65-69
Foster, John M.; Thoma, Brent P.; Heard, Richard W. (2004) Range extensions and review of the caprellid amphipods (Crustacea: Amphipoda; Caprellidae) from the shallow coastal waters from the Suwannee River, Florida, to Port Aransas, Texas, with an illustrated key, Gulf and Caribbean Research 16(2): 161-175
Galil, B. S. (2009) Taking stock: inventory of alien species in the Mediterranean sea., Biological Invasions 11: 359-372
Griffiths, Charles L. (1976) <missing title>, South African Museum, Cape Town. Pp. 106
Guerra-Garcia, J. M. (2004) The Caprellidea (Crustacea, Amphipoda) from Western Australia and Northern Territory, Australia, Hydrobiologia 522: 1-74
Guerra-García, J. M. and 7 authors (2011) Geographical expansion of the invader Caprella scaura (Crustacea: Amphipoda: Caprellidae) to the East Atlantic coast, Marine Biology 158: 2617-2622
Guerra-Garcia, J. M.; Takeuchi, I. (2004) The Caprellidea (Crustacea: Amphipoda) from Tasmania., Journal of Natural History 38: 967-1044
Guerra-Garcia, J. M.; Takeuchi, I (2003) The Caprellidea (Malacostraca: amphipoda) from Mirs Bay, Hong Kong, with the description of a new genus and two new species., Journal of Crustacean Biology 23(1): 154-168
Guerra-García, José M. (2003) The Caprellidea (Crustacea: Amphipoda) from Mauritius Island, Western Indian Ocean, Zootaxa 232: 1-24
Guerra-García, José Manuel; de Figueroa, José Manuel Tierno (2009) What do caprellids (Crustacea: Amphipoda) feed on?, Marine Biology 156: 1881-1890
Hosono, Takashi; Munehara, Hirayuki (2001) Caprellids (Crustacea, Amphipod, Caprellidea) from Usujiri, Pacific coast of southern Hokkaido, Bulletin of Fisheries Sciences, Hokkaido University 52(1): 11-37
Huang, Zongguo (Ed.), Junda Lin (Translator) (2001) Marine Species and Their Distributions in China's Seas, Krieger, Malabar, FL. Pp. <missing location>
Krapp, Traudi; Lang, Carola; Libertini; Melzer, Roland R. (2006) Caprella scaura Templeton, 1836 sensu lato (Amphipoda: Caprellidae) in the Mediterranean., Organisms, Diversity and Evolution 3: 3-18
Lacerda, Mariana Baptista; Masunari, Setuko (2011) [Identification key for caprellids (Crustacea, Amphipoda) from coast of Paraná and Santa Catarina states.], Biota Neotropica 11(3): Published online
Laubitz, Diana R. (1991) Crustacea Amphipoda Caprellidae: Caprellids from the western Pacific (New Caledonia, Indonesia, and the Philippines)., Memoires du Museum Nationale d'Histoire Naturelle 152: 101-123
Laubitz, Diana R. (1995) Caprellidea (Crustacea: Amphipoda) from the southern and western Indian Ocean., Mesogee 54: 61-100
Lim, S. T. A.; Alexander, C. G. (1986) Reproductive behavior of the caprellid Caprella scaura typica Mayer 1890, Marine Behavior and Physiology 12: 217-230
Liu, Wenliang; Liang, Xiaoli ; Zhu, Xiaojing (2015) A new record and mitochondrial identification of Synidotea laticauda Benedict, 1897 (Crustacea: Isopoda: Valvifera: Idoteidae) from the Yangtze Estuary, China, Zootaxa 4294: 371-380
Lowry, J. K.; Stoddart, H. E. (2003) <missing title>, 19.2B CSIRO Publishing, Canberra, Australia. Pp. <missing location>
Marelli, Dan C. (1981) New records for Caprellidae in California, and notes on morphological variant of Caprella verrucosa Boek, 1871, Proceedings of the Biological Society of Washington 94(3): 654-662
Martínez-Laiz, G.; Ros, M.; Guerra-García, J. M. ; Faasse, M.; Santos, A. M.;; Cabezas, M. P. (2021) Using molecular data to m.onitor the post-establishment evolution of the invasive skeleton shrimp Caprella scaura, Marine Biodiveristy 166(105266): Published online
Martínez-Laiz, G.;· Guerra?García, J. M.; · Ros, M.; Fenwick, D.; Bishop, J. D.; Horton, T.; Faasse, M. A.; Cabezas, M. P. (2021) Hitchhiking northwards: on the presence of the invasive skeleton shrimp Caprella scaura in the UK, Marine Biodiveristy 51(78): Published online
Martínez, Julián; Adarraga, Idoia (2008) First record of invasive caprellid Caprella scaura Templeton, 1836 sensu lato (Crustacea: Amphipoda: Caprellidae) from the Iberian Peninsula., Aquatic Invasions 3(2): 164-170
McCain, John C. (1968) The Caprellidae (Crustacea, Amphipoda) of the Western North Atlantic, Bulletin of the United States National Museum 278: 1-115
Montalto L, Ezcurra de Drago I (2003) Tolerance to desiccation of an invasive mussel, Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae), under experimental conditions, Hydrobiologia 498(1): 161–167
https://doi.org/10.1023/A:1026222414881
National Benthic Infaunal Database 2012-2014 National Benthic Infaunal Database (NBID). <missing URL>
Occhipinti Ambrogi, Anna (2000) Biotic invasions in a Mediterranean lagoon., Biological Invasions 2: 165-176
Parretti, Paola; Ros, Macarena; Gestoso, Ignacio; Ramalhosa, Patrício; Costa, Ana Cristina; Canning-Clode, João (2021) Assessing biotic interactions between a non-indigenous amphipod and its congener in a future climate change scenario, Aquatic Invasions 16: 186-207
Pires-Teixeira, Larissa M. Neres-Lima, Vinicius Creed, Joel C. (2021) How do biological and functional diversity change in invaded tropical marine rocky reef communities?, Diversity 13(353): Published online
Prato, Ermelinda; Parlapiano, Isabella; Biandolino, Francesca (2013) Seasonal fluctuations of some biological traits of the invader Caprella scaura (Crustacea: Amphipoda: Caprellidae) in the Mar Piccolo of Taranto (Ionian Sea, southern Italy), Scientia Marina 77(1): 169-178
Ramalhosa, Patrício; Canning-Clode, João (2015) The invasive caprellid Caprella scaura Templeton, 1836 (Crustacea: Amphipoda: Caprellidae) arrives on Madeira Island, Portugal, BioInvasions Records 4(2): 97-102
Revanales, Triana; Guerra-García, José M.; Ros, Macarena (2022) Colonization dynamics of potential stowaways inhabiting marinas: Lessons from caprellid crustaceans, Water 14(2659.): Published online
doi.org/10.3390/w14172659
Rifi, Mouna; Basti, Leila; Rizzo, Lucia; Tanduo, Valentina; Radulovici, Adriana; Jaziri, Sabri; Uysal, Irfan; Souissi, Nihel; Mekki, Zeineb; Crocetta. Fabio (2023) Tackling bioinvasions in commercially exploitable species through interdisciplinary approaches: A case study on blue crabs in Africa’s Mediterranean coast (Bizerte Lagoon, Tunisia, Estuarine, Coastal and Shelf Science 291(108419): Published online
https://doi.org/10.1016/j.ecss.2023.108419
Rodríguez-Almaraz, Gabino A.; Ortega-Vidales, Víctor M. (2013) [First record of Caprella scaura and Caprella penantis (Crustacea: Peracarida: Amphipoda) in the Laguna Madre, Tamaulipas, Mexico], Revista Mexicana de Biodiversidad 84: 989-993
Ros, Macarena and 6 authors (2014) Exploring trophic strategies of exotic caprellids (Crustacea: Amphipoda): Comparison between habitat types and native vs introduced distribution ranges, Estuarine, Coastal and Shelf Science 139: 88-98
Ros, Macarena; Vazquez-Luis, Maite; Guerra-Garcia, Jose Manuel (2013) The tropical caprellid amphipod Paracaprella pusilla: a new alien crustacean in the Mediterranean Sea, Helgoland Marine Research 67: 675-685
Ros, Macarena; Guerra-García, José M.; González-Macías, Manuel; Saavedra, Ángela; López-Fe, Carlos M. L (2013) Influence of fouling communities on the establishment success of alien caprellids (Crustacea: Amphipoda) in Southern Spain, Marine Biology Research 9(3): 261-273
Ros, Macarena; Lacerda, Mariana B.; Vazquez-Luis, Maite; Masunari, Setuko; Guerra-Garc?a, Jose´ M. (2016) Studying exotics in their native range: Can introduced fouling amphipods expand beyond artificial habitats?, Biological Invasions Published online: <missing location>
Ros, Macarena; Vázquez-Luis, Maite; Guerra-García, José M. (2013) The role of marinas and recreational boating in the occurrence and distribution of exotic caprellids (Crustacea: Amphipoda) in the Western Mediterranean: Mallorca Island as a case study, Journal of Sea Research 83: 94-103
Ros, Macarena; Vázquez-Luis, Maite; Guerra-García, José M. (2015) Environmental factors modulating the extent of impact in coastal invasions: The case of a widespread invasive caprellid (Crustacea: Amphipoda) in the Iberian Peninsula, Marine Pollution Bulletin 98: 247-258
Salmon, Terry and 21 authors 2014-2022 California Fish Website. https://calfish.ucdavis.edu/
Sano, Minoru; Omori, Michio; Taniguchi, Kazuya (2003) Predator-prey systems of drifting seaweed communities off the Tohoku coast, northern Japan, as determined by feeding habit analysis of phytal animals, Fisheries Science 69: 260-268
Santos, Cinthya S. G. (2007) Nereididae from Rocas Atoll (North-East, Brazil)., Arq. Mus. Nac., Rio de Janeiro 65(3): 369-380
Tremblin, Clement M.; Holzmann, Maria; Parker, Justin H.; Sadekov, Aleksey; Haig, David W. (2022) Invasive Japanese foraminifera in a south-west Australian estuary, Marine and Freshwater Research 73: 328–342
https://doi.org/10.1071/MF21254
Turcotte, Christian; Sainte Marie, Bernard (2009) Biological synopsis of the Japanese skeleton shrimp Caprella mutica, Canadian Manuscript Report of Fisheries and Aquatic Sciences 2903: 1-26
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/
Virgili. Riccardo; Tandua, Valentina; Katsanevakis, Stelos; Terlizzi, Francesco; Villani, Guido; Fontano. Angelo; Crocetta, Fabio (2022) The Miseno Lake (Central-Western. Mediterranean Sea): An overlooked reservoir of non-indigenous and cryptogenic ascidians in a marine reserve, Frontiers in Marine Science 9(866906): Published online
Watling, Les; Carlton, James T. (2007) The Light and Smith Manual: Intertidal invertebrates from Central California to Oregon (4th edition), University of California, Berkeley CA. Pp. 618-629
Zenetos, A.; Vassilopoulou, V.; Salomidi, M.; Poursanidis, D. (2008) Additions to the marine alien fauna of Greek waters (2007 update)., Marine Biodiversity Records 1: e91