Invasion History
First Non-native North American Tidal Record: 1924First Non-native West Coast Tidal Record:
First Non-native East/Gulf Coast Tidal Record: 1924
General Invasion History:
Corophium volutator is native to the Atlantic coasts of Europe, from southern Norway to Spain (Crawford 1937; Bousfield 1973; Lincoln 1979). Records from the Mediterranean and Black Sea probably refer to the closely related C. orientale, formerly regarded as a variety or subspecies of C. volutator (Bousfield and Hoover 1997). Corophium volutator is tolerant of brackish and 'nearly fresh' waters (Bousfield 1973). It ranges through the Baltic Sea into the 2-3 PSU isohaline of the Gulf of Bothnia (Dobrzycka and Szaniawska 1995). On the East Coast of the U.S., its range is limited to the Gulf of Maine, from Boston to the Bay of Fundy. Genetic analysis indicates that the population in the Gulf of Maine is introduced (Einfeldt and Addison 2015). The first published reports of C. volutator are by Huntsman and Sparks (1924, cited by Einfeldt and Addison 2015). Blake (1933) and Shoemaker (1934a) referred to specimens taken in the Bay of Fundy, and Maine. Although this species was not discovered in the Gulf of Maine until the early 20th century, it has been studied as a keystone species in Gulf of Maine mudflats, as an ecosystem engineer and a vital link in local food webs (Wilson and Parker 1997; Percy 1999; Hamilton et al. 2006).
North American Invasion History:
Invasion History on the East Coast:
Corophium volutator was not collected in extensive late-19th century surveys of the U.S. Fish Commission in the Gulf of Maine. The earliest published mentions of this amphipod in the Gulf of Maine are from a survey of the Mt. Desert Island region, based on collections from 1926-1932 (Blake 1933). Shoemaker (1934a) mentions a specimen collected near Pine Point, Scarborough, Maine (ME), and several collections by the Biological Board of Canada, in the mouths of rivers entering the Bay of Fundy. Blake (1933) described C. volutator as 'abundant on certain mud flats'. Bousfield (1973) gave its range as Casco Bay (ME) through the Bay of Fundy, but it has also been reported from islands in Boston Harbor (before 2001, Bell et al. 2005). By the 1980s, it was recognized as an ecologically important species as a food organism for invertebrates, fishes, and shorebirds, and as a deposit feeder in the Bay of Fundy (e.g., Comitto 1982; Murdoch 1986).
Earlier authors noted the disparity in the range of Corophium volutator on the east and west sides of the Atlantic (Crawford 1937; Shoemaker 1947; Bousfield 1973). Chapman (2000) listed C. volutator as a probable introduction on the East Coast of North America. Wilson (1997) cited an unpublished, preliminary study suggesting a long genetic divergence between the two populations. A recent genetic study found that the genotypes of North American populations were a subset of European genetic diversity (Einfeldt and Addison 2015). The genetic data best support the model of two separate introductions to the lower Gulf of Maine and the Bay of Fundy. Since C. volutator is abundant in intertidal mud, dry ballast appears to be the likeliest mechanism for transport, although this amphipod also swims frequently, so ballast water cannot be ruled out (Einfeldt and Addison 2015).
Description
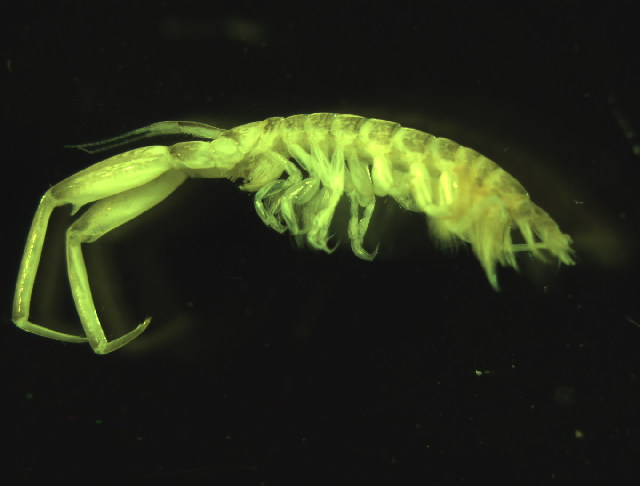
Corophium volutator has a slender, very depressed body, and a massive Antenna 2, especially in the male, which is longer than Antenna 1. The coxal plates are small and separated. The urosome segments are not fused. Both sexes have a short, triangular rostrum, with the tip about equal to that of the eye-lobes. In dorsal view, the inner margins of segment 1 are crenulated in the male, and the flagellum is long, with 10-12 segments. Segments 2 and 4 of Antenna 2 each have a stout distal spine, while segment 5 is of equal length to 4, but lacks a distal spine. On Gnathopod 1, segment 6 is shorter than segment 5, and its palm is transverse and evenly convex. For Gnathopod 2, segments 5 and 6 are both shorter than segment 2, and the dactyl is long, lacking teeth. Pereiopods 3 and 4 have their bases a little inflated, and the dactylus is shorter than the propodus and carpus combined. Periopods 5-7 have increasing densities of setae on the posterior margins of the bases and pereiopod 7 is strongly elongated. All three uropods are biramous, but the inner ramus of Uropod 3 is greatly reduced. The peduncle of Uropod 1 has 10-12 spines on the outer margin and 3-4 long spines on the inner margin. The peduncle of Uropod 2 has 3-5 dorsal spines. Adults are 5-6 mm long, excluding Antenna 2. The body is tan, mottled with dark brown in the interior of the body segments and larger appendage segments. This description is based on Bousfield 1973, Lincoln 1979, and Bousfield and Hoover 1997.
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Arthropoda | |
| Subphylum: | Crustacea | |
| Class: | Malacostraca | |
| Subclass: | Eumalacostraca | |
| Superorder: | Peracarida | |
| Order: | Amphipoda | |
| Suborder: | Gammaridea | |
| Family: | Corophiidae | |
| Genus: | Corophium | |
| Species: | volutator |
Synonyms
Oniscus volutator (Pallas, 1766)
Potentially Misidentified Species
Corophium orientale Schellenberg 1928 is a closely related species found in the Mediterranean, Black, and Azov Seas (Bousfield and Hoover 1997). It was formerly regarded as a subspecies or variety of C. volutator (e.g., Crawford 1937). Mediterranean and Black Sea records probably all refer to this species. Corophium orientale has been collected on the Atlantic Coast of Spain, in the Guadalquivir Estuary, where it was considered a probable introduction (Cuesta et al. 1996).
Ecology
General:
Gammarid amphipods have separate sexes, brooded embryos, and direct development (Bousfield 1973). Juveniles are about 1 mm in length, and the sexes become distinguishable at about 5 mm. Females tend to be larger than males, reaching a maximum of 12 mm, versus 8.5 mm for males (Barbeau et al. 2010; Percy 1999). (NB: Lengths are from rostrum to telson and exclude the antenna). In the Bay of Fundy, the sex ratio is strongly skewed towards females, while in most other locations it is closer to 1:1. Males leave the burrow and crawl or swim in search of females. A male may visit many burrows before entering one and mating (Percy 1999). The eggs and young are brooded by the female. Broods range from 10 to 172 eggs, averaging about 38, and tend to increase with the females' size. Broods are larger in the Bay of Fundy than at other locations (Mills and Fish 1979; Percy 1999). Broods take about 14 days, one tidal cycle, to develop, and tend to be born on a rising tide (Mills and Fish 1979). Populations in the lower Bay of Fundy, Northern Scotland, and the Baltic have only one generation per year, while those in Maine, England, western Sweden, and the upper Bay of Fundy have two generations, possibly because of higher water temperatures (Wilson and Parker 1996; Percy 1999). Central Maine populations breed in May-June and again in August-September (Wilson and Parker 1996).
Corophium volutator, as an inhabitant of mudflats and marshes with wide tidal ranges, and much air exposure, is tolerant of very wide ranges of temperature and salinity. It can survive in intertidal mud at temperatures below 0°C, with most animals in the upper 15 mm of sediment, although mortality occurs steady throughout the winter, probably due to depletion of fat reserves (Drolet et al. 2013). This amphipod has been found in fresh or 'nearly fresh' water (Bousfield 1973; Buckley 2004). In experiments, 50% of animals survived 10 days in fresh water, while 65% survived at very high salinities of 50 PSU. In the Gulf of Bothnia, Baltic Sea, it occurs up to the 3-4 PSU isohaline (Dobrzycka, and Szaniawska 1995). Corophium volutator builds a U-shaped burrow, first inspecting the surface and then choosing sediments with the right mix of fine and coarse particles for an easily dug, but stable burrow. The burrows often penetrates anoxic sediment (Meadows 1966). While this amphipod is mostly a burrow-dweller, many individuals, especially males and juveniles, also actively swim (Drolet et al. 2013). While C. volutator is usually thought of as an intertidal animal, in the Baltic, where tidal range is minimal, this amphipod occurs in and around seagrass beds down to 5 m (Bostrom et al. 2006).
This amphipod feeds on detritus and benthic microalgae (mostly diatoms), on the sediment surface, but can also capture suspended particles and phytoplankton. In experiments, when offered water with dense phytoplankton, C. volutator grazed until the cell concentration dropped to a threshold level, and then switched to scraping the sediment surface (Riisgard and Schotge 2007). Because of its frequent high abundance in estuaries, it has a major role as a grazer, burrower, and food organism. Major predators include polychaetes, fishes, and shorebirds (Comitto 1982; Percy 1999; MacDonald et al. 2014).
Food:
Detritus; Phytoplankton
Consumers:
Fishes; shorebirds
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Unstructured Bottom | None |
| General Habitat | Salt-brackish marsh | None |
| General Habitat | Grass Bed | None |
| Tidal Range | Subtidal | None |
| Tidal Range | Low Intertidal | None |
| Tidal Range | Mid Intertidal | None |
| Vertical Habitat | Endobenthic | None |
| Vertical Habitat | Epibenthic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | -2 | Field. Corophium volutator survived short-term (48 h) severe cold temperatures (-8 °C), and can survive 2 weeks or more at -3 °C (Drolet et al. 2013). |
| Maximum Temperature (ºC) | 39 | Experimental, at 30 ppt (Mills and Fish 1980) |
| Minimum Salinity (‰) | 0.2 | Field Data, Buckley 2004, Stour estuary, England; Experimental data, Dobrzycka, and Szaniawska 1995 |
| Maximum Salinity (‰) | 50 | Experimental data, Dobrzycka, and Szaniawska 1995 |
| Minimum Length (mm) | 4.8 | Females, Nova Scotia (Barbeau et al. 2010). Males are distinguishable at ~ 5 mm (Percy 1999). |
| Maximum Length (mm) | 10.5 | Females, Nova Scotia (Barbeau et al. 2010) |
| Broad Temperature Range | None | Cold temperate-Warm temperate |
| Broad Salinity Range | None | Tidal Limnetic-Hyperhaline |
General Impacts
Although the amphipod Corophium volutator appears to be a relatively new arrival in the Gulf of Maine, it appears to have become locally abundant on mudflats, and in the Bay of Fundy, plays the role of a keystone species and an ecological engineer. Populations in the Bay of Fundy mudflats graze a large portion of the available microalgae, and provide food for 2-3 million migrating birds, and for fishes on the mudflats. The benthic microalgae, mostly diatoms, secrete carbohydrates which stabilize the sediment surface, so that amphipod feeding results in the destabilization of the sediment. Shorebird and fish predation reduces the amphipod population, and results in restoration of benthic diatoms and re-stabilization of the sediment (Hawkins 1985; Percy 1999; Daborn et al. 2002; Hamilton 2006).Economic impacts of C. volutator are difficult to estimate, but the huge numbers of migrating shorebirds supported by this amphipod are valued aesthetically by tourists, birders, and conservationists, not just in the Bay of Fundy, but along much of the East Coast (Percy 1999). In addition, the fishes feeding on Corophium in the mudflats (Atlantic Shiner and Tomcod) (Salinas 1980, cited by Hawkins 1985) are small, and are likely forage for larger, commercially important fishes.
Regional Impacts
| NA-ET2 | Bay of Fundy to Cape Cod | Ecological Impact | Bioturbation | ||
| On a Bay of Fundy mudflat, bioturbation by Corophium volutator decreases the cohesion of sediment during periods of submersion. The cohesion of sediments is primarily due to carbohydrates secreted by diatoms. Predation by huge flocks of migrating shorebirds (Semipalmated Sandpipers, Calidris pusilla) drastically reduces C. volutator populations, and results in increased diatom populations and increased sediment stability (Daborn et al. 1993). | |||||
| N030 | Narraguagus Bay | Ecological Impact | Food/Prey | ||
| Corophium volutator is a major prey item for the Clamworm Alitta virens in mudflats near Jonesboro, Maine. The predatory polychaete controls the abundance of C. volutator in this system (Comitto 1982). | |||||
| NA-ET2 | Bay of Fundy to Cape Cod | Ecological Impact | Food/Prey | ||
| In the Gulf of Fundy, Corophium volutator plays a major role as a food organism for birds and fishes, particularly 2-3 million migrating Semipalmated Sandpipers (Calidris pusilla), and other shorebirds, which feed on mudflats while migrating to the Arctic (Hawkins 1985; Percy 1999; Daborn et al. 2002; Hamilton et al. 2006; MacDonald et al. 2012). It is also a major food item for fishes (Atlantic Silverside, Menidia menidia and Atlantic Tomcod, Microgadus tomcod) in the Bay of Fundy, accounting for 29 to 94% of the fishes' gut contents in May-July (Salinas 1980, cited by Hawkins 1985). Its role in the carbon budget of Bay of Fundy mudflats is as link between the primary producers (benthic diatoms and phytoplankton) and larger consumers such a s birds and fishes (Hawkins 1985).
Corophium volutator is a major prey item for the Clamworm Alitta virens in mudflats near Jonesboro, Maine. The predatory polychaete controls the abundance of C.volutator in this system (Comitto 1982). |
|||||
| NA-ET2 | Bay of Fundy to Cape Cod | Ecological Impact | Trophic Cascade | ||
| Corophium volutator is part of a cascade of indirect effects, in which feeding by Semipalmated Sandpipers reduces the bioturbation and diatom grazing by the amphipods. The removal of the amphipods results in recovery of the diatoms and their secretions, which result in increased stability of the sediment (Percy 1999; Daborn et al. 2002)/ | |||||
| NA-ET2 | Bay of Fundy to Cape Cod | Ecological Impact | Herbivory | ||
| Corophium volutator is a major grazer in intertidal mudflats and shallow estuaries, feeding on benthic microalgae (mostly diatoms), suspended phytoplankton, and detritus (Percy 1999; Riisgard and Schotge 2007). This amphipod consumed an estimated 27% of microalgal production on the Peck's Cove mudflat, New Brunswick. Grazing by C. volutator lowers the chlorophyll content of the surface sediments until late summer, when bird and fish predation reduced the amphipod population (Hawkins 1985). | |||||
| ME | Maine | Ecological Impact | Food/Prey | ||
| Corophium volutator is a major prey item for the Clamworm Alitta virens in mudflats near Jonesboro, Maine. The predatory polychaete controls the abundance of C. volutator in this system (Comitto 1982). | |||||
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Appeltans, W. et al. 2011-2015 World Registry of Marine Species. <missing URL>Barbeau, Myriam A.; Grecian, Lorelei A.;Arnold, Erin E.; Sheahan, Deirdre C.; Hamilton, Diana J. (2009) Spatial and temporal variation in the population dynamics of the intertidal amphipod Corophium volutator in the upper Bay of Fundy, Canada, Journal of Crustacean Biology 29(4): 491-506
Bell, Richard; Buchsbaum, Robert; Chandler, Mark (2005) Inventory of intertidal marine habitats, Boston Harbor Islands National Park area, Northeastern Naturalist 12(Special Issue 3): 169-200
Blake, Charles H. (1933) Marine Fauna, Biological Survey of the Mount Desert Island Region 5: 1-290
Borza, Peter ; Arbaciauskas, Kestutis Zettler, Michael L. (2021) Multidimensional niche differentiation might buffer invasion impacts: the case of oligohaline corophiids (Crustacea: Amphipoda) in the Baltic Sea, Biological Invasions 23: 18191-1900
Bostrom, Christoffer; Lastuniemi, Maria; Bonsdorff, Erik (2006) Infaunal responses to seagrass habitat structure: A study of life-history traits and population dynamics of Corophium volutator (Pallas), Marine Biology Research 2: 398-410
Bousfield, E. L.; Hoover, P. M. (1997) The amphipod superfamily Corophioidea on the Pacific coast of North America. Part V. Family Corophiidae: Corophiinae, new subfamily. Systematics and distributional ecology., Amphipacifica 2(3): 67-139
Bousfield, E.L. (1973) <missing title>, Comstock Publishing Associates, Ithaca, NY. Pp. <missing location>
Bubinas, Algis; Vaitonis, Gintautas (2003) The analysis of the structure, productivity, and distribution of zoobenthocenoses in the Lithuanian economic zone of the Baltic Sea and the importance of some benthos species to fish diet, Acta Zoologica Lituanica 13(2): 114-124
Buckley, Phil; Dussart, George; Trigwell, Jacqueline (2004) Invasion and expansion of Corophiidae (Amphipoda) in the Stour estuary (Kernt, UK)., Crustaceana 77(4): 425-433
Chapman, John W. (2000) Marine Biological Invasions; Proceedings of the first national conference, January 24-27, 1999., MIT Sea Grant College Program, Cambridge MA. Pp. 66-80
Comitto, J. A. (1982) Importance of predation by infaunal polychaetes in controlling the structure of a soft-bottom community in Maine, USA, Marine Biology 68: 77-81
Crawford, G. I. (1937) A review of the amphipod genus Corophium, with notes on the British species., Journal of the Marine Biological Association of the United Kingdom 21: 589-630
Cuesta, J.A.; Serrano, L.; Bravo, M. R.; Toja, J. (1996) Four new crustaceans in the Guadalquivir River estuary (SW Spain), including an introduced species., Limnetica 12(1): 41-45
Daborn, Graham R.; Amos, Carl L.; Brylinsky, Michael; Christian, Harold; Drapeau, Georges (2002) An ecological cascade effect: Migratory birds affect stability of intertidal sediments, Limnology and Oceanography 38(1): 225-231
Dobrzycka, Aldona; Szaniawska, Anna (1995) The effect of salinity on osmoregulation in Corophium volutator (Pallas) and Saduria entomon (Linnaeus) from the Gulf of Gdansk, Oceanologia 37(1): 111-122
Drolet, David; Kennedy, Kelan; Barbeau, Myriam A. (2013) Winter population dynamics and survival strategies of the intertidal mudflat amphipod Corophium volutator (Pallas), Journal of Experimental Marine Biology and Ecology 441: 126-137
Drolet, David; Barbeau, Myriam A. (1999) Diel and semi-lunar cycles in the swimming activity of the intertidal, benthic amphipod Corophium volutator in the upper Bay of Fundy, Canada, Crustaceana 29(1): 51-56
Drolet, David; Barbeau, Myriam A. (2012) Population structure of resident, immigrant, and swimming Corophium volutator (Amphtipoda) on an intertidal mudflat in Canada, Journal of Sea Research 20: 1-13
Eddy,Elizabeth N. ; Roman, Charles T. (2016) Relationship between epibenthic invertebrate species assemblages and environmental variables in Boston Harbor’s intertidal habitat, Northeastern Naturalist 23(1): 45-66
Einfeldt, A. L.; Addison, J. A. (2013) Hydrology influences population genetic structure and connectivity of the intertidal amphipod Corophium volutator in the northwest Atlantic, Marine Biology 160: 1015-1027
Einfeldt, Anthony L.; Addison, Jason A. (2015) Anthropocene invasion of an ecosystem engineer: resolving the history of Corophium volutator (Amphipoda: Corophiidae) in the North Atlantic, Biological Journal of the Linnean Society 115: 288-304
Fish, J. D.; Mills, A. (1979) The reproductive biology of Corophium volutator and C. arenarium (Crustacea: Amphipoda), Journal of the Marine Biological Association of the United Kingdom 59: 335-358
Gerdol, Veronika; Hughes, R. G. (1994) Feeding behaviour and diet of Corophium volutator in an estuary in southeastern England, Marine Ecology Progress Series 114: 103-108
Hamilton, Diana J.; Diamond, Antony W.; Wells, Peter G. (2006) Shorebirds, snails, and the amphipod (Corophium volutator) in the upper Bay of Fundy: top-down vs. bottom–up factors, and the influence of compensatory interactions on mudflat ecology, Hydrobiologia 567: 285-306
Hawkins, C. W. (1985) Population carbon budgets and the importance of the amphipod Corophium volutator in the carbon transfer on a Cumberland Bay mudflat, upper Bay of Fundy, Canada, Netherlands Journal of Sea Research 19(2): 165-176
Jarv, Leili; Kotta, Jonne; Kotta, Ilmar; Raid, Tiit (2011) Linking the structure of benthic invertebrate communities and the diet of native and invasive fish species in a brackish water ecosystem., Annales Zoologici Fennici 48: 129-141
Jazdzewski, Krzysztof; Konopacka, Alicja (1993) Survey and distribution of Crustacea Malacostraca in Poland, Crustaceana 65(176-191): <missing location>
Kater, Belinda; Jol, Johan G.; Smit, Mathijs G. D. (2008) Growth of Corophium volutator under laboratory conditions, Archives of Environmental Contamination and Toxicology 54: 440-446
Larsen, Peter F.; Doggett, Lee F. (1991) The macroinvertebrate fauna associated with the mud flats of the Gulf of Maine, Journal of Coastal Research 7(2): 365-375
Lawrie, Sarah M.; Raffaelli, David G.; Emes, Charles H. (2000) Small-scale patterns in the distribution of the amphipod Corophium volutator on the Ythan estuary, Aberdeenshire, Scotland, Marine Biology Research 85: 321-327
Lincoln, Roger J. (1979) British Marine Amphipoda: Gammaridea., In: (Eds.) . , London. Pp. <missing location>
MacDonald, Elizabeth C.; Frost, Elisabeth H.; MacNeil, Stephanie M.; Hamilton, Diana J.; Barbeau, Myriam A. (2014) Behavioral response of Corophium volutator to shorebird predation in the upper Bay of Fundy, Canada, None 9(10): e110633
MarLin- Marine Life Information Network 2006-2024 MarLin- Marine Life Information Network. <missing URL>
Meisner, Karin; Bick, Andreas (1997) Population dynamics and ecoparasitological surveys of Corophium volutator in coastal waters in the Bay of Mecklenburg (southern Baltic Sea), Diseases of Aquatic Organisms 29: 169-179
Murdoch, Mary; Barlocher, Felix; Laltoo, Mary (1986) Population dynamics and nutrition of Corophium volutator (Pallas) in the Cumberland Basin, Bay of Fundy, Journal of Experimental Marine Biology and Ecology 103: 235-249
Nakano D, Kobayashi T, Sakaguchi I (2010a) Differences in larval dynamics of golden mussel Limnoperna fortunei between dam reservoirs with and without an aeration system, Landscape and Ecological Engineering 6(1): 53–60
https://doi.org/10.1007/s11355-009-0082-7
Pelegri, S. R; Blackburn, T. H. (1994) Bioturbation effects of the amphipod Corophium volutator on microbial nitrogen transformations in marine sediments, Marine Biology 121: 253-258
Percy, J. A. 1999 Keystone <em>Corophium</em>: Master of the mudflats. <missing URL>
Percy, J. A. 2003 Alien Invasions: introduced species to the Bay of Fundy and environs. <missing URL>
Riisgård, Hans Ulrik; Schotge, Peer (2007) Surface deposit feeding versus filter feeding in the amphipod Corophium volutator, Marine Biology Research 3(6): 421-427
Shoemaker, Clarence R. (1934a) The amphipod genus Corophium on the east coast of America, Proceedings of the Biological Society of Washington 47: 23-32
Shoemaker, Clarence R. (1947) Further notes on the amphipod genus Corophium, from the east coast of North America, Journal of the Washington Academy of Sciences 37(2): 47-63
Trott, Thomas J. (2004) Cobscook Bay inventory: a historical checklist of marine invertebrates spanning 162 years., Northeastern Naturalist 11(Special issue 2): 261-324
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/
Wilson, A.B., Boates, J.S., Snyder, M. (1997) Genetic isolation of populations of the gammaridean amphipod, Corophium volutator, in the Bay of Fundy, Canada, Molecular Ecology 6: 917-923
Wilson, W. Herbert Jr.; Parker, Kristian (1996) The life history of the amphipod Corophium volutator: the effects of temperature and shorebird predation, Journal of Experimental Marine Biology and Ecology 196: 239-250
Yale Peabody Museum of Natural History 2008-2016 YPM Invertebrate Zoology - Online Catalog. <missing URL>