Invasion History
First Non-native North American Tidal Record: 1953First Non-native West Coast Tidal Record: 1953
First Non-native East/Gulf Coast Tidal Record:
General Invasion History:
Tenellia adspersa was described from Odessa, Ukraine and is now widely distributed around the world, most likely spread by shipping (Roginskaya 1970; Thompson and Brown 1984). It is native in the Eastern Atlantic, from Europe (Norway to Portugal and Spain) and the Western Mediterranean (Roginskaya 1970). In the Baltic, it occurs eastward to the Gulf of Finland (Roginskaya 1970; Evertsen et al. 2004). In much of its European range, it is confined to brackish lagoons (Thompson and Brown 1984).
On the East Coast of North America, the range and invasion status of T. adspersa is unclear, owing to frequent confusion with a very similar native species, T. fuscata (Gould 1870), which has an overlapping range (Marcus 1972; Chester 1996). It has been introduced to Brazil, the West Coast of North America, Japan, and the Caspian Sea (Roginskaya 1970; Antsulevich and Starobogatov 1990). It feeds on many species of hydroids and tolerates a wide range of salinity and temperature (Roginskaya 1970). Hydroid prey include a number of global invaders: Cordylophora caspia, Garveia franciscana, and Ectopleura crocea (Roginskaya 1970). The mode of larval development is variable according the adult's nutritional status, between lecithotrophic development, with a short planktonic period, and planktotrophic development, with a longer planktonic phase (Chester 1996), more favorable for ballast water transport.
North American Invasion History:
Invasion History on the West Coast:
Tenellia adspersa was first found on the West Coast at Point Richmond, San Francisco Bay in 1953 (Jones 1954, cited by Carlton 1979). It was subsequently found at many locations within the Bay, including Berkeley Yacht Harbor in 1963 (Steinberg 1963); Lake Merritt Oakland in 1970 (Carlton 1979); San Leandro in 1993 (Cohen and Carlton 1995); Sausalito around 1995 (Cohen and Carlton 1995), and the Napa River in 2004 (Cohen et al. 2005). It was found on hydroids in Monterey Bay (Steinberg 1963), but was not seen there after 1958 (Carlton 1979). However, it was found in Elkhorn Slough in 1977 (Carlton 1979), and was established by 1998 (Wasson et al. 2001). It was also found in Alamitos Bay, in Long Beach in 1968 (Carlton 1979). In 1986, T. adspersa was found in upper Coos Bay, Oregon (Goddard 1990).
Invasion History on the East Coast:
Tenellia adspersa was first reported from the East Coast in 1969, based on collections made in Chesapeake Bay, starting in 1969 (Marcus 1972). However, its status in the Northwest Atlantic is complicated by confusion with the very similar T. fuscata, described from the Charles River estuary, Massachusetts, which overlaps in range and habitat (Marcus 1972; Vogel 1977). Consequently, we regard T. adspersa as cryptogenic in the Northwest Atlantic, until the status and distribution of the two species is resolved. At least one population, initially identified as T. fuscata, in Great Bay, New Hampshire (Gaulin et al. 1986) was later found to be T. aspersa (Chester 1996). Currently, T. adspersa is known from Great Bay, New Hampshire to Beaufort, South Carolina (Marcus 1972; Eyster 1979; Chester 1996; Caine 1998), while T. fuscata is reported from Maine to Chesapeake Bay (Gould 1870; Franz 1968), with one report from the Indian River Lagoon, Florida (Clark 1995). Vogel (1977) reported both species from Chesapeake Bay, with T. adspersa tending to occur at lower salinities (average 12.5 PSU) than T. fuscata (14.5-30 PSU). On Smithsonian Environmental Research Center (SERC) fouling plates in Chesapeake Bay, Terrence Gosliner found only T. adspersa, but only a few animals were examined (Ruiz et al., unpublished data).
Invasion History Elsewhere in the World:
Tenellia adspersa was described from Odessa, Ukraine on the Black Sea in 1845, but was not found in the Sea of Azov until 1963 (Roginskaya 1970). It could have been overlooked, or transported by ships. Increased salinity in the Sea, due to hydroelectric dams, could have favored the invasion. Its introduction to the Caspian Sea in 1990 was clearly due to transport in ballast water, by ships traversing the canal from the Black Sea (Antsulevich and Starobogatov 1990).
Beyond Europe, it was introduced to brackish waters in Brazil before 1953 (Vannucci and Hosoe 1953, cited by Roginskaya 1970). One specimen was found in the Seto Inland Sea, Japan in 1959 (Baba and Hamatani 1963). More recently, it was found in a bypass canal in Vladivostok, Russia, on the Sea of Japan in 1995, but did not become established (Zvyagintsev et al. 2011). One record is known from Botany Bay, Sydney, Australia in 1988 (Australian Museum Malacology C.155082, Atlas of Living Australia 2014), but establishment is unknown.
Description
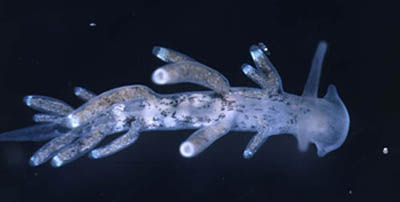
Tenellia adspersa has a slender body and a rounded head with lateral lobes, sometimes prolonged into oral tentacles, resulting in a crescent shape. The rhinophores (dorsal head tentacles) are smooth and cylindrical. There are 2 to 6 rows of cerata (dorsal outgrowths containing nematocysts) across the back, each with 1-3 cerata on each side of the body. The penis has an apical stylet. The animal rarely exceeds 8 mm in length. The overall color is yellowish to pale brown in color with black stippling over the body. Description based on Roginskaya (1970), Marcus (1972), Vogel (1977), Thompson and Brown (1984), and Behrens (1991).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Mollusca | |
| Class: | Gastropoda | |
| Subclass: | Opisthobranchia | |
| Order: | Nudibranchia | |
| Family: | Tergipedidae | |
| Genus: | Tenellia | |
| Species: | adspersa |
Synonyms
Embletonia grayi (Saville-Kent, 1869)
Embletonia pallida (Alder and Hancock, 1854)
Eolis ventilabrum (Dalyell, 1853)
Tenellia mediteranea (Costa, 1866)
Tenellia pallida (Thompson and Brown, 1976)
Tenellia ventilabrum (Dalyell, 1853)
Tergipes adspersa (Nordmann, 1845)
Tergipes lacinulatus (Schultze, 1849)
Potentially Misidentified Species
Calliopaea bellula (Stiliger bellulus) is an herbivorous sacoglossan native to the Northeast Atlantic, from Norway to the Mediterranean and Black Seas (Rudman 2009). Tenellia adspersa in the Sea of Azov was originally identified as this animal (Roginskaya 1970).
Stiliger fuscata
Stiliger fuscata is native to the Northwest Atlantic from New Hampshire to Florida. It is an herbivorous sacoglossan, found on algae, but superficially resembles Tenellia spp. However, the cerata are concentrated in the posterior part of the body (Abbott 1974).
Stiliger fuscovittata
Stiliger fuscovittatus is native to the Northeast Pacifiic from Alaska to Mexico. It has been introduced to Florida and Texas, but has not become established. It is an herbivorous sacoglossan, found on algae, but superficially resembles Tenellia spp. However, S. fuscovittatus lacks the crescent-shaped pair of lobes on the head, and is marked with two parallel reddish-brown streaks on the body (Behrens 1991).
Tenellia fuscata
Tenellia fuscata is very similar to T. adspersa, but differs by lacking a penial stylet and having a hermaphroditic valve (Chambers 1934, cited by Franz 1968; Marcus 1972). It has been reported from Massachusetts to Florida (Gould 1870; Franz 1968; Clark 1995). Careful morphological examination and genetic analysis may be needed to determine the distribution of the two species of Tenellia spp. and confirm their status as separate species.
Ecology
General:
Tenellia adspersa is a small nudibranch associated with hydroids, often in brackish water. Nudibranchs are simultaneous hermaphrodites and copulate reciprocally or unilaterally. Tenellia adspersa lays its eggs in an oval gelatinous mass containing 3-40 eggs, attached to hydroid stalks (Roginskaya 1970). The mode of development of the eggs varies with the nutritional status of the adults. This species can produce direct-developing larvae, lecithotrophic larvae (<1 day in plankton), and planktotrophic larvae (4-5 days in plankton) (Eyster 1979; Thompson and Brown 1984). Chester (1996) found that development time at 20°C was 5-6 days for eggs of continuously fed animals, but decreased to 3 days for starved animals. Both the type of larva produced and the rate of development appear to be variable between and within populations. This non-genetic variation in larval development is unusual in invertebrates, and is known as 'poeciligony'.
Tenellia adspersa has been reported from lagoons, estuaries, and harbors of warm-temperate to tropical regions. Substrates include rocks, pilings, floating docks, and boat hulls (Steinberg 1963; Roginskaya 1970; Chester 1996). This nudibranch tolerates an unusually wide range of salinity, from 3 to 50 PSU (Roginskaya 1970; Harris 1980), although some populations show reduced reproduction below 10-12 PSU (Harris et al. 1980; Blezard 1999). Tenellia adspersa feeds on a very wide range of hydroids, including Cordylophora caspia, Garveia franciscana, Ectopleura crocea, Gonothyraea loveni, Obelia spp., Protohydra leukarti, Eudendrium sp., and Bougainvillea sp. (Roginskaya 1970; Vogel 1977; Eyster 1979; Harris et al. 1980; Thompson and Brown 1984; Goddard 1990; Caine 1998). The feeding rate can be quite high. One T. adspersa can consume 100 Garveia hydranths in a day (Turpaeva 1963, cited by Roginskaya 1970). Chester et al. (2000) reported lower rates, up to seven C. caspia polyps eaten per day. Experiments indicate that T. adspersa can survive long periods of time without feeding, so that they can prevent the regrowth of hydroids after extensive predation (Chester et al. 2000). In many nudibranchs, the cnidocysts (stinging cells) of hydroids are ingested and then incorporated into the cerata, as a defensive mechanism (Barnes 1983). This is probably the case in T. adspersa.
Food:
Hydroids
Trophic Status:
Carnivore
CarnHabitats
| General Habitat | Grass Bed | None |
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Oyster Reef | None |
| General Habitat | Marinas & Docks | None |
| Salinity Range | Mesohaline | 5-18 PSU |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 4 | Lowest tested (Harris et al. 1980) |
| Maximum Temperature (ºC) | 25 | Field (Eyster 1979) |
| Minimum Salinity (‰) | 3 | Lab experiments, cited by Roginskaya (1970) |
| Maximum Salinity (‰) | 50 | Lab experiments, cited by Roginskaya (1970) |
| Minimum Reproductive Salinity | 10 | Experiments cited by Roginskaya (1970) |
| Maximum Reproductive Salinity | 50 | Lab experiments, (Harris et al. 1980) |
| Minimum Duration | 0 | Development appears to vary with adult feeding and condition; well-fed adults lay eggs which develop directly, hatching out juveniles. Lower levels of feeding yield lecithotrophic or planktotrophic larvae (Chester 1996). All three modes of development can be seen simultaneously in a population (Eyster 1979; ) |
| Maximum Duration | 4.9 | Planktotrophic larvae hatched from adults fed at lower levels (Chester 1996). |
| Minimum Length (mm) | 5 | Roginskaya 1970 |
| Maximum Length (mm) | 8 | Roginskaya 1970 |
| Broad Temperature Range | None | Cold-Temperate-Warm-Temperate |
| Broad Salinity Range | None | Oligohaline-Polyhaline |
General Impacts
Tenellia adspersa is of ecological and economic interest because of its predation on a wide range of fouling hydroids, and because its ease of culture permits study of predator-prey relations (Roginskaya 1970; Harris et al. 1980; Gaulin et al. 1986; Chester et al. 2000).Economic Impacts
High abundances of Tenellia sp. have been reported on fouling plates near power plants in Chesapeake Bay (Cory 1967; Abbe 1987) and in the Sea of Azov (Roginskaya 1970). Tenellia adspersa might have some beneficial impact on fouling in cooling systems if its predation reduced biomasses of hydroids such as Garveia franciscana and Cordylophora caspia (Chester et al. 2000).
Ecological Impacts
Predation: Tenellia adspersa is potentially an important predator on a wide range of hydroids, including widely introduced Garveia franciscana (Rope Grass Hydroid) and Cordylophora caspia (Freshwater Hydroid) (Roginskaya 1970; Harris et al. 1980). In experimental cultures, T. adspersa's chemical cues and predation caused an alteration of C. caspia's growth form, resulting in a greater density of hydranths. This could limit later settlement of T. adspersa's larva, due to predation. Tenellia adspersa predation on C. caspia thus seems to have complex effects; small numbers of T. adspersa might actually increase C. caspia biomass (Gaulin et al. 1986). However, modelling predicts that under a wide range of conditions, T. adspersa will eliminate C. caspia colonies, resulting in succession of hydroids by other fouling organisms (Chester et al. 2000).
Regional Impacts
| N130 | Great Bay | Ecological Impact | Predation | ||
| Predation by Tenellia adspersa on the hydroid Cordylophora caspia stimulated increased growth and budding in the hydroid, resulting in a denser colony, perhaps favoring the persistence of the colonies (Gaulin et al. 1986). However, population modeling, including reproduction and settlement, indicates that T. adspersa is likely to remove colonies of C. caspia, favoring succession in the fouling community (Chester et al. 2000). | |||||
| S110 | Broad River | Ecological Impact | Predation | ||
| Tenellia adspersa was observed feeding on the hydroid Bougainvillia rugosa. However, the rate of predation was reduced by interference from the caprellid Paracaprella tenuis, which flicked the nudibranch away (Caine 1998). | |||||
| CAR-VII | Cape Hatteras to Mid-East Florida | Ecological Impact | Predation | ||
| Tenellia adspersa was observed feeding on the hydroid Bougainvillia rugosa. However, the rate of predation was reduced by interference from the caprellid Paracaprella tenuis, which flicked the nudibranch away (Caine 1998). | |||||
| NA-ET2 | Bay of Fundy to Cape Cod | Ecological Impact | Predation | ||
| Predation by Tenellia adspersa on the hydroid Cordylophora caspia stimulated increased growth and budding in the hydroid, resulting in a denser colony, perhaps favoring the persistence of the colonies (Gaulin et al. 1986). However, population modeling, including reproduction and settlement, indicates that T. adspersa is likely to remove colonies of C. caspia, favoring succession in the fouling community (Chester et al. 2000). | |||||
| SC | South Carolina | Ecological Impact | Predation | ||
| Tenellia adspersa was observed feeding on the hydroid Bougainvillia rugosa. However, the rate of predation was reduced by interference from the caprellid Paracaprella tenuis, which flicked the nudibranch away (Caine 1998). | |||||
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Abbe, George R. (1987) Epifauna, In: Heck, Kenneth L.(Eds.) Ecological studies in the middle reach of Chesapeake Bay- Calvert Cliffs. , Berlin. Pp. 82-91Abbott, R. Tucker (1974) American Seashells, Van Nostrand Reinhold, New York. Pp. <missing location>
Agarwal, Robin Gwen 2023 After the algal bloom cleared out of Lake Merritt, the nudibranchs came to party in droves. https://baynature.org/2023/05/30/after-the-algal-bloom-cleared-out-of-lake-merritt-the-nudibranchs-came-to-party-in-droves/
Aladin, Nikolai V.; Plotnikov, Igor S.; Filipov, Andrei A. (2002) Invasive aquatic species of Europe: Distribution, impacts, and management., Kluwer Academic Publishers, Dordrecht. Pp. 351-359
Antsulevich, A. E.; Starobogatov, Y. I. (1990) First finding of the nudibranch mollusk (order, Tritoniformes) in the Caspian Sea, Zoologicheskii Zhurrnal 69(1): 138-140
Associated Press (12/2021) Lummi Nation declares disaster after invasive crab arrives, Seattle Times <missing volume>: <missing location>
Atlas of Living Australia 2013-2016 Atlas of Living Australia. <missing URL>
Baba, Kikutaro; Hamatani, Iwao (1963) A short account of the species Tenellia pallida (A. & H.) taken from Mukashima, Japan (Nudibranchia-Eolidoidea), Publications of the Seto Marine Biological Laboratory 11(2): 167-168
Barnes, Robert D. (1983) Invertebrate Zoology, Saunders, Philadelphia. Pp. 883
Behrens, D.W. (1991) Pacific Coast Nudibranchs, In: (Eds.) . , Monterey, California. Pp. <missing location>
Blezard, David J. (1999) <missing title>, M.S. Thesis, University of New Hampshire, Durham, New Hampshire. Pp. <missing location>
Caine, Edsel A. (1998) First case of capreild amphipod-hydrozoan mutualism, Journal of Crustacean Biology 18(2): 317-320
Carlton, James T. (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific Coast of North America., Ph.D. dissertation, University of California, Davis. Pp. 1-904
Chester, Charles M. (1996) The effect of adult nutrition on the reproduction and development of the estuarine nudibranch, Tenellia adspersa (Nordmann, 1845), Journal of Experimental Marine Biology and Ecology 198: 113-130
Chester, Charles M.; Turner, Roy; Carle, Michael; Harris, Larry G. (2000) Life history of a nudibranch/hydroid association: A discrete event simulation, Veliger 43(4): 338-348
Chukhchin, V. D. (1984) {Ecology of gastropod mollusks of the Black Sea}, In: (Eds.) . , <missing place>. Pp. <missing location>
Clark, Kerry B. (1995) Rheophilic/oligotrophic lagoonal communities through the eyes of slugs (Mollusca: Opisthobranchia), Bulletin of Marine Science 57(1): 242-251
Cohen, Andrew N. and 10 authors (2005) <missing title>, San Francisco Estuary Institute, Oakland CA. Pp. <missing location>
Cohen, Andrew N.; Carlton, James T. (1995) Nonindigenous aquatic species in a United States estuary: a case study of the biological invasions of the San Francisco Bay and Delta, U.S. Fish and Wildlife Service and National Sea Grant College Program (Connecticut Sea Grant), Washington DC, Silver Spring MD.. Pp. <missing location>
Cory, Robert L. (1967) Epifauna of the Patuxent River Estuary, Chesapeake Science 8(2): 71-89
Evertsen, Jussi; Bakken, Torkild (2005) Nudibranch diversity (Gastropoda, Heterobranchia) along the coast of Norway, Fauna Norvegica Series A 25: 1-37
Evertsen, Jussi; Bakker, Torkild; Green, Sandra (2004) Rediscovery of Tenellia adspersa (Nudibranchia) from the Finnish Archipelago, Sarsia 89: 363-365
Eyster, L. S. (1979) Reproduction and developmental variability in the Opisthobranch Tenellia pallida, Marine Biology 51: 133-140
Eyster, Linda S. (1980) Distribution and reproduction of some shell-less opisthobranchs from South Carolina, Bulletin of Marine Science 30(3): 580-599
Farrapeira, Cristiane Maria Rocha; Tenório, Deusinete de Oliveira ; do Amaral, Fernanda Duar (2011) Vessel biofouling as an inadvertent vector of benthic invertebrates occurring in Brazil, Marine Pollution Bulletin 62: 832-839
Franz, David R. (1968) Occurrence and distribution of New Jersey Opisthobranchia, Nautilus 82(1): 7-12
Gaulin, Gary; Dill, Lauren; Beaulieu, Juli; Harris, Larry G. (1986) Predation-induced changes in growth form in a nudibranch hydroid association., Veliger 28(4): 389-393
Goddard, Jeffrey H. R. (1990) Additional opisthobranch mollusks from Oregon, with a review of deep-water records and observations of the fauna of the south coast, Veliger 33(3): 230-237
Gould, Augustus A. (1870) <missing title>, Wright and Potter, State Printers, Boston. Pp. <missing location>
Harris, Larry G.; Powers, Mark; Ryan, Julia (1980) Life history studies of the estuarine nudibranch Tenellia fuscata (Gould 1870), Veliger 23(1): 70-74
Marcus, Eveline du Bois Reymond (1972) Notes on some opisthobranch gastropods from the Chesapeake Bay, Chesapeake Science 13(4): 300-317
Marcus, Eveline du Bois Reymond (1977) An annotated checklist of the western Atlantic warm water opisthobranchs, Journal of Molluscan Studies Supplement: 1-22
McDonald, Gary R. (2007) The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon, University of California Press, Berkeley CA. Pp. 788-807
MIT Sea Grant 2003-2008 Introduced and cryptogenic species of the North Atlantic. <missing URL>
MIT Sea Grant 2009-2012 Marine Invader Tracking and Information System (MITIS). <missing URL>
Roberts D, Wells FE (1980) The marine and estuarine molluscs of the Albany area of Western Australia, Records of the Western Australia Museum 8: 335–357
Roginskaya, I. S. (1970) Tenellia adspersa, a nudibranch new to the Azov Sea, with notes on its taxonomy and ecology, Malacological Review 3: 167-174
Rosenberg, Gary 1995-2023 Malacolog 4.1. http://www.malacolog.org/
Rudman, W. B. 1997-2016 Sea Slug Forum. http://www.seaslugforum.net/
Ruiz, Gregory M.; Geller, Jonathan (2018) Spatial and temporal analysis of marine invasions in California, Part II: Humboldt Bay, Marina del Re, Port Hueneme, and San Francisco Bay, Smithsonian Environmental Research Center & Moss Landing Laboratories, Edgewater MD, Moss Landing CA. Pp. <missing location>
Steinberg, Joan (1960) Rare and little-known opisthobranch mollusks from the West Coast of North America, Veliger 3(2): 49
Steinberg, Joan (1963) Notes on the opisthobranchs of the West Coast of North America- IV. A distributional list of opisthobranchs from Point Conception to Vancouver Island, Veliger 6(2): 69-72
Thompson, Michelle Lynne (1993) <missing title>, M.S. Thesis, College of William and Mary, Williamsburg VA. Pp. <missing location>
Thompson, T. E. (1976) <missing title>, Ray Society, London. Pp. <missing location>
Thompson, T. E.; Brown, G. H. (1984) <missing title>, Ray Society, London. Pp. <missing location>
Vogel, Rosalie M. (1977) <missing title>, M.S. Thesis, College of William and Mary, Williamsburg, VA.. Pp. <missing location>
Wasson, Kerstin; Zabin, C. J.; Bedinger, L.; Diaz, M. C.; Pearse J. S. (2001) Biological invasions of estuaries without international shipping: the importance of intraregional transport, Biological Conservation 102: 143-153
Zvyaginstev, A. Yu.; Radashevsky, V. I.; Ivin, V. V.; Kashin, I. A.; Gorodkov, A. N. (2011) Nonindigenous species in the far-eastern seas of Russia, Russian Journal of Biological Invasions 2(1): 164-182