Invasion History
First Non-native North American Tidal Record: 1877First Non-native West Coast Tidal Record: 1915
First Non-native East/Gulf Coast Tidal Record: 1877
General Invasion History:
The precise origin of Styela plicata is unknown. The type specimen was described from a ship in the Delaware River, Philadelphia, Pennsylvania in 1823 (Van Name 1912). It was apparently well established south of Cape Hatteras by the late 19th and early 20th centuries before regular collecting began. It is believed to have been introduced to the Mediterranean, probably centuries ago (Monniot, in Food and Agricultural Organization 2000). James T. Carlton considers the species introduced to the Northwest Atlantic, based on the general diversity of this genus in the Pacific (Carlton and Rucklelshaus 1997; Carlton, pers. comm.). Genetic analysis by de Barros et al. (2009) suggested the Northwest Pacific as the probable native region. However, another study (Pineda et al. 2011) found the highest genetic diversity in the Western Atlantic, although the authors could not definitely identify a native region. The cladistic history of the genus Styela does support a Northwest Pacific ancestry of S. plicata, since it is distinct both from an Eastern Atlantic clade of warm-shallow water Styela, and cold deep-water forms (e.g. S. rustica (Carlton et al., in prep.)). Pineda et al's (2011) genetic analysis is consistent with historical data that suggests that transport of S. plicata began very early, and was very frequent, blurring genetic distinctions among native and introduced populations.
North American Invasion History:
Invasion History on the West Coast:
In the Northeast Pacific, Styela plicata was first collected in San Diego Bay in 1915 and ranges north to Santa Barbara, California (Lambert and Lambert 1998; Nichols et al. 2023). To the south, it has been reported from Baja California, Mexico, being collected in Ensenada in 2000 (Lambert and Lambert 2003) and Bahia San Quintin in 2005 (Rodriguez and Ibarra-Obando 2008).
Invasion History on the East Coast:
The type specimen for Styela plicata was described from a ship in the Delaware River, Philadelphia, Pennsylvania in 1823 (Van Name 1912; 1945). However, because this specimen was reported from a vessel hull and not as present in the surrounding environment, we do not consider this record to be its date of introduction. It was also collected in 1880 on Blackfish Bank, off Charleston, South Carolina (USNM 6960, U.S. National Museum of Natural History 2007). The long-established range of S. plicata along the Western Atlantic is from Cape Lookout, North Carolina (USNM 14790, U.S. National Museum of Natural History 2007) to Padre Island, Texas (USNM 14424, U.S. National Museum of Natural History 2007), and south through the Caribbean to Venezuela (Van Name 1945; USNM 14481, U.S. National Museum of Natural History 2007). However, S. plicata has been collected north of Cape Hatteras, in Lynnhaven Bay, Virginia Beach, at the mouth of Chesapeake Bay (Ruiz et al., unpublished data), in Chincoteague Bay, Virginia (in 1999, O'Beirn et al. 2004, on oyster-culture floats), and in the Mystic River Estuary, in the Long Island Sound in Mystic Connecticut, where thousands of juveniles to adults were found attached to many different substrates in the fall of 2022. The previous appearance of S. plicata in the Mystic River Estuary was in the fall of 2001, when a lone specimen was attached to a the bottom of a local resident boat and collected (J.T. Carlton, personal communication). It appears from careful monitoring that no individuals survived the winter from 2022–2023, and no further specimens were found in 2023 and 2024. The Mystic CT population is thus considered extinct, although S. plicata remains on the radar as a highly probably future invader in New England (J.T. Carlton, personal communication).
Invasion History on the Gulf Coast:
A specimen of Styela plicata was collected from Padre Island, Texas (USNM 14424, U.S. National Museum of Natural History 2007) and Cedar Key, Florida in 1877 (USNM 940, U.S. National Museum of Natural History 2007). This tunicate is established and abundant on the Gulf Coast from Florida through Texas (Van Name 1921; Van Name 1945; Ruiz et al., unpublished data).
Invasion History Elsewhere in the World:
The long-established range of Styela plicata along the Western Atlantic is from Cape Lookout, North Carolina (USNM 14790, U.S. National Museum of Natural History 2007) to Padre Island, Texas (USNM 14424, U.S. National Museum of Natural History 2007), and south through the Caribbean to Venezuela (Van Name 1945; USNM 14481, U.S. National Museum of Natural History 2007). In 1884, S. plicata was reported from St. Thomas, US Virgin Islands/Caribbean Sea (USNM 6916, U.S. National Museum of Natural History 2007), and from Bermuda in 1882 (USNM 2769, U.S. National Museum of Natural History 2007) suggesting that it was established and widespread in warm waters of the Western Atlantic by the late 19th century.
Styela plicata was collected from Panama in 1973 (USNM 19744, US National Museum of Natural History 2007). Nearly a century before, in 1883, it was collected from Rio de Janeiro state, Brazil (Trautstedt 1883, cited by da Rocha and Kremer 2005), It ranges south to Uruguay (Orensanz et al. 2002), but in Bahia, northern Brazil, it was known only from 'one individual' (da Rocha and Kremer 2005).
Styela plicata is considered introduced to the Mediterranean: ['This species is not native to the Mediterranean, but was introduced centuries ago. It is present in all warm-temperate and tropical regions, especially in zones of human activity.'] (C. F. Monniot, in Food and Agricultural Organization 2000). It was collected from Naples (Traustedt 1877, cited by Kott 1985) and Trieste, Italy (Heller 1877, cited by Kott 1998). Outside the Mediterranean, it has been collected from Dakar, Senegal (Monniot 1969).
In the Northwest Pacific, S. plicata ranges from Hong Kong (Huang 2001) to Mutsu Bay, at the north end of Honshu, Japan (Oka 1935, cited by Nishikawa 1991). Within the Indian Ocean, it has been collected from Vizhinjam, India, on the Arabian Sea (Abdul and Sivakumar 2007), Somalia (in 1964, USNM 18297, U.S. National Museum of Natural History 2007), and the Gulf of Suez (in 1927, Monniot 2002). These scattered records could represent introductions.
In the Southwest Pacific, S. plicata was first collected at Port Jackson, near Sydney, Australia (Heller 1878, cited by Kott 1985). Its range runs from the mouth of the Calliope River, Queensland to Port Phillip Bay (in 1963, Millar 1966, cited by Keough and Ross 1999), and west to Cockburn Sound and the Perth area (Hartmeyer and Michaelsen 1928, cited by Kott 1985). Within this range, it is strongly associated with harbors and artificial structures (Kott 1985; Keough and Ross 1999). A genetic survey indicates high diversity in Australian populations, with significant genetic structure in more southern latitudes, but no structure in tropical latitudes (David et al. 2010). In New Zealand, it has been reported at several locations on the North Island, including Auckland Harbour and Hauraki Gulf (in 1957, Cranfield et al. 1998), Gulf Harbour Marina (Inglis et al. 2005), and Taranaki (Inglis et al. 2006).
Description
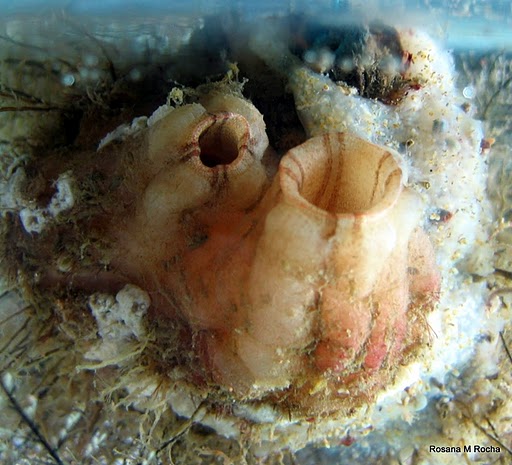
Styela plicata is a solitary tunicate, variable in shape, but roughly oval. It is fixed to the substrate by the posterior end of its body, usually without roots or stalks. Its tunic is firm and thick, slightly translucent, with deep, irregular, longitudinal furrows, and horizontal creases that form large, irregularly rounded lumps. Its total body length can reach 90 mm. The oral siphon is terminal, and the atrial siphon is a little behind it – both siphons are short, with square apertures with rounded humps on each side. The color of the tunic is whitish with brown or black stripes radiating from the siphons (Van Name 1945; Kott 1985; Nishikawa 1991; Gretchen Lambert, personal communication 2012).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Chordata | |
| Subphylum: | Tunicata | |
| Class: | Ascidiacea | |
| Order: | Stolidobranchia | |
| Family: | Styelidae | |
| Genus: | Styela | |
| Species: | plicata |
Synonyms
Styela gyrosa (Heller, 1883)
Styela pinguis (Herdman, 1899)
Tethyum plicatum (Hartmeyer, 1909)
Ascidia cuvieri (Delle Chiaje, 1841)
Ascidia patata (Costa, 1844)
Ascidia phusca (Delle Chiaje, 1828)
Phallusia sulcata (Delle Chiaje, 1841)
Potentially Misidentified Species
New species intorduced to Caribbean Panama and Venezuela, of unkknown origin (de Barros and da Rocha 2021).
Styela panamensis
New species, native to Caribbean Panama. Records of S. plicata from Bocas del Toro, Panama, may refer to S. panamensis.
Ecology
General:
Life History- A solitary tunicate is ovoid, elongate or vase-like in shape, with two openings or siphons. Most solitary tunicates attach to substrates by their side or base, but some attach with a conspicuous stalk. They are sessile filter feeders with two siphons, an oral and an atrial siphon. Water is pumped in through the oral siphon, where phytoplankton and detritus is filtered by the gills, and passed on mucus strings to the stomach and intestines. Waste is then expelled in the outgoing atrial water.
Solitary ascidians are hermaphroditic, meaning that both eggs and sperm are released to the atrial chamber. Eggs may be self-fertilized or fertilized by sperm from nearby animals, but many species have a partial block to self-fertilization. Depending on the species, eggs may be externally or internally fertilized. In external fertilizers, eggs and sperm are released through the atrial siphon into the surrounding water column were fertilization takes place. In internal fertilizers, eggs are brooded and fertilized within the atrial chamber and then released into the water column upon hatching. Fertilized eggs hatch into a tadpole larva with a muscular tail, notochord, eyespots, and a set of adhesive papillae. The lecithotrophic (non-feeding, yolk-dependent) larva swims briefly before settlement. Swimming periods are usually less than a day and some larvae settle immediately after release, but the larval period can be longer at lower temperatures. Once settled, the tail is absorbed, the gill basket expands, and the tunicate begins to feed by filtering (Barnes 1983).
Food:
Phytoplankton
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Marinas & Docks | None |
| General Habitat | Oyster Reef | None |
| General Habitat | Vessel Hull | None |
| General Habitat | Canals | None |
| General Habitat | Mangroves | None |
| General Habitat | Rocky | None |
| General Habitat | Salt-brackish marsh | None |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
Life History
Tolerances and Life History Parameters
| Maximum Temperature (ºC) | 30.2 | Field, US East & West Coast marinas (Lord et al. 2015) |
| Minimum Salinity (‰) | 17.5 | Experimental- This was tested experimentally with animals at Santa Barbara CA. Only '75% seawater' (26 ppt), '50% seawater' (18ppt), and '110% seawater' (38.5 ppt) were used (Sims 1984). |
| Maximum Salinity (‰) | 40 | Field salinity (Shark Bay, Western Australia) (Wyatt et al. 2005) |
| Minimum Reproductive Temperature | 18 | Experimental- Lowest temperature tested for embryonic development and metamorphosis (Thiyagarajan and Qian 2003). |
| Maximum Reproductive Temperature | 30 | Experimental- Highest temperature tested for embryonic development and metamorphosis. Only 30% of larvae successfully completed development (Thiyagarajan and Qian 2003). |
| Minimum Reproductive Salinity | 30 | Experimental- Development was observed at 22, 26, 30, and 34 ppt. Embryonic development was unsuccessful at 22 and 26 ppt (Thiyagarajan and Qian 2003). |
| Maximum Reproductive Salinity | 34 | Experimental- Highest salinity tested (Thiyagarajan and Qian 2003). |
| Minimum Duration | 0 | Larvae can attach immediately after hatching (Thiyagarajan and Qian 2003). |
| Maximum Duration | 2 | Larvae prevented from settling (Thiyagarajan and Qian 2003). |
| Maximum Length (mm) | 90 | Van Name 1945 |
| Broad Temperature Range | None | Warm temperate-Tropical |
| Broad Salinity Range | None | Polyhaline-Euhaline |
General Impacts
Economic Impacts
Fisheries: Styela plicata is known to foul cultured bivalves, interfering with their growth in Brazil, Hong Kong, Japan, and Spain (da Rocha et al. 2009).
Ecological Impacts
Competition: Styela plicata has been a dominant fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It dominates fouling communities from Ensenada, Mexico to Santa Barbara (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes from southern California harbors (Lambert and Lambert 1998). Styela plicata was one of the dominant fouling organisms on fouling plates at Beaufort, North Carolina. It invaded plates initially dominated by other species and created 'monopolies,' for up to four months (Sutherland and Karlson 1977). Settled juveniles of Styela plicata inhibited settlement of the native Microcosmus squamiger (from Brisbane, Australia), in a laboratory experiment (Ruis et al. 2009). The mechanism was not clear, but could involve competition for food or allelopathy (inhibitory chemicals).
Fisheries: Styela plicata is known to foul cultured bivalves, interfering with their growth in Brazil, Hong Kong, Japan, and Spain (da Rocha et al. 2009). On the positive side, S. plicata is extensively cultured on long lines in Korea and Japan (Lambert et al. 2016).
Regional Impacts
| NEP-VI | Pt. Conception to Southern Baja California | Ecological Impact | Competition | ||
| Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It dominates fouling communities from Ensenada, Mexico to Santa Barbara (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P020 | San Diego Bay | Ecological Impact | Competition | ||
| Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It produced large monospecific patches in several locations during serveral years in San Diego Bay (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P030 | Mission Bay | Ecological Impact | Competition | ||
| Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It produced large monospecific patches in several locations during serval years in San Diego Bay (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P023 | _CDA_P023 (San Louis Rey-Escondido) | Ecological Impact | Competition | ||
| Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It produced large monospecific patches in Oceanside Harbor (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P027 | _CDA_P027 (Aliso-San Onofre) | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P040 | Newport Bay | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P050 | San Pedro Bay | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P060 | Santa Monica Bay | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P062 | _CDA_P062 (Calleguas) | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P064 | _CDA_P064 (Ventura) | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P065 | _CDA_P065 (Santa Barbara Channel) | Ecological Impact | Competition | ||
| It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| CAR-VII | Cape Hatteras to Mid-East Florida | Ecological Impact | Competition | ||
| Styela plicata was one of the dominant fouling organisms on fouling plates at Beaufort, North Carolina. It invaded plates initially dominated by other species and created 'monopolies'; for up to four months (Sutherland and Karlson 1977). | |||||
| S030 | Bogue Sound | Ecological Impact | Competition | ||
| Styela plicata was one of the dominant fouling organisms on fouling plates at Beaufort, North Carolina. It invaded plates intially dominated by other species and created 'monopolies; which lasted for up to four months (Sutherland and Karlson 1977). | |||||
| MED-II | None | Ecological Impact | Competition | ||
| Fouling cultured bivalves in the Ebro Delta, Spain (Perera et al. 1990, cited by da Rocha et al. 2009). | |||||
| MED-II | None | Economic Impact | Fisheries | ||
| Fouling cultured bivalves in the Ebro Delta, Spain (Perera et al. 1990, cited by da Rocha et al. 2009). | |||||
| NWP-2 | None | Ecological Impact | Competition | ||
| Fouls cultured bivalves in Hong Kong (Huang 2003, cited by da Rocha et al. 2009). | |||||
| NWP-2 | None | Economic Impact | Fisheries | ||
| Fouls cultured bivalves in Hong Kong (Huang 2003, cited by da Rocha et al. 2009). | |||||
| SA-II | None | Ecological Impact | Competition | ||
| Fouls cultured oysters (Crassostrea gigas) and mussels (Perna perna) in farms in Santa Catarina, Brazil (da Rocha et al. 2009). | |||||
| SA-II | None | Economic Impact | Fisheries | ||
| Fouls cultured oysters (Crassostrea gigas) and mussels (Perna perna) in farms in Santa Catarina, Brazil (da Rocha et al. 2009). | |||||
| NWP-3b | None | Ecological Impact | Competition | ||
| Fouls cultured oysters (Crassostrea gigas) in Hiroshima Bay (Arakawa 1990, cited by da Rocha et al. 2009). | |||||
| NWP-3b | None | Economic Impact | Fisheries | ||
| Fouls cultured oysters (Crassostrea gigas) in Hiroshima Bay (Arakawa 1990, cited by da Rocha et al. 2009). | |||||
| AUS-XII | None | Ecological Impact | Competition | ||
| Settled juveniles of Styela plicata inhibited settlement of the native Microcosmus squamiger (from Brisbane, Australia), in a laboratory experiment. The mechanism was not clear, but could involve competition for food or allelopathy (inhibitory chemicals; Rius et al. 2009). | |||||
| CA | California | Ecological Impact | Competition | ||
| Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It produced large monospecific patches in several locations during serveral years in San Diego Bay (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It produced large monospecific patches in several locations during serval years in San Diego Bay (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Styela plicata has been the dominant marina fouling organism in southern California harbors since 1960 (Lambert and Lambert 1998). It produced large monospecific patches in Oceanside Harbor (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| NC | North Carolina | Ecological Impact | Competition | ||
| Styela plicata was one of the dominant fouling organisms on fouling plates at Beaufort, North Carolina. It invaded plates intially dominated by other species and created 'monopolies; which lasted for up to four months (Sutherland and Karlson 1977). | |||||
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map


References
Orth, Donald J. (2010) Socrates Opens a Pandora’s Box of Northern Snakehead Issues, American Fisheries Society Symposiums 89: 203-221Abbott, Donald P.; Johnson, Jeffrey V. (1972) The ascidians Styela barnharti, S. plicata, and S. montereyensis in Californian waters., Bulletin of the Southern California Academy of Sciences 71: 95-105
Abdul, Jaffar Ali H.; Sivakumar, V. (2007) Occurrence and distribution of ascidians in Vizhinjam Bay (south west coast of India)., Journal of Experimental Marine Biology and Ecology 342: 189-190
Agius, C.; Schembri, P. J.; Jaccarini, V. (1977) A preliminary report on organsims fouling oyster cultures in Malta., Memorie di Biologia Marina e di Oceanographia 7(3-4): 51-59
Airoldi, Laura; Turon, Xavier; Perkol-Finkel, Shimrit; Rius, Marc (2015) Corridors for aliens but not for natives: effects of marine urban sprawl at a regional scale, Diversity and Distributions 21: 755-768
Australian Museum Business Services (2002) <missing title>, Australian Museum Business Services, for Sydney Ports Corporation, Sydney. Pp. <missing location>
Barnes, P. B.; Roberts, D. E.; Davis, A. R. (2013) Biodiversity in saline coastal lagoons: patterns of distribution and human impacts on sponge and ascidian assemblages, Diversity and Distributions 19: 1394-1406
Barnes, Robert D. (1983) Invertebrate Zoology, Saunders, Philadelphia. Pp. 883
Bastida-Zavala, Rolando; de León-González, Jesús Ángel; Carballo Cenizo, José Luis; Moreno-Dávila, Betzabé (2014) [Aquatic Invasive Species in Mexico], Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, <missing place>. Pp. 317-336
Beshai, Ryan A.; Truong, Danny A.; Henry, Amy K. Sorte, Cascade J. B. (2022) Biotic resistance or invasional meltdown? Diversity reduces invasibility but not exotic dominance in southern California epibenthic communities, Biological Invasions 25(2): 533-549
https://doi.org/10.1007/s10530-022-02932-1
Bonvechio, Kimberly I.; Barthel, Brandon; Carroll, Jessica (2018) Health and Genetic Structure of the American Eel in Florida, Southeastern Naturalist 17(3): 483-455
https://doi.org/10.1656/058.017.0311
California Department of Fish and Wildlife (2014) Introduced Aquatic Species in California Bays and Harbors, 2011 Survey, California Department of Fish and Wildlife, Sacramento CA. Pp. 1-36
Cangussu, Leonardo C. and 5 authors (2010) Substrate type as a selective tool against colonization by non-native sessile invertebrates, Brazilian Journal of Oceanography 58(3): 219-231
Carlton, James T. (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific Coast of North America., Ph.D. dissertation, University of California, Davis. Pp. 1-904
Carlton, James T. (2000) Marine bioinvasions ecology in the 21st century, In: Pederson, Judith(Eds.) Marine Bioinvasions. , Cambridge MA. Pp. 6-23
Carlton, James T.; Ruckelshaus, Mary H. (1997) Nonindigenous marine invertebrates and algae of Florida, In: Simberloff, Daniel, Schmitz, Don C., Brown, Tom C.(Eds.) Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida. , Washington, D.C.. Pp. 187-201
Chainho, Paula and 20 additional authors (2015) Non-indigenous species in Portuguese coastal areas, lagoons, estuaries, and islands, Estuarine, Coastal and Shelf Science <missing volume>: <missing location>
Cooley, Nelson R. (1978) An inventory of the estuarine fauna in the vicinity of Pensacola, Florida., Florida Marine Research Publications 31: 1-119
Cornelio, Michele; Manzoni, Alberto (1999) Caratterizzazione stagionale degli insediamenti di organismi macrobentonici su substrati sperimentali nel bacino centrale della laguna di Venezia., Bollettino del Museo Civico di Storia Naturale di Venezia 49: 135-144
Cranfield, H.J.; Gordon, D.P.; Willan, R.C.; Marshall, B.A; Battershill, C.N.; Francis, M.P.; Nelson, W.A.; Glasby, C.J.; Read, G.B. (1998) <missing title>, The National Institute of Water and Atmospheric Research, New Zealand. Pp. <missing location>
Currie, D. R.; McArthur, M. A.; Cohen, B. F. (1999) Exotic Marine Pests in the Port of Geelong, Victoria, In: Hewitt, Campbell, Thresher & Martin(Eds.) Marine Biological Invasions of Port Phillip Bay, Victoria. , Hobart, Tasmania. Pp. 227-246
da Rocha, Rosana M.; Costa, Luciana (2005) Ascidians (Urochordata: Ascidiacea) from Arriaal do Cabo, Rio de Janeiro, Brazil., Iheringia Series Zoologie 95(1): 57-64
da Rocha, Rosana M.; Faria, Suzana B.; Moreno, Tatiane R. (2005) Ascidians from Bocas del Toro, Panama, Caribbean Journal of Science 41(3): 600-612
da Rocha, Rosana M.; Kremer, Laura P.; Baptista, Mariah S.; Metri, Rafael (2009) Bivalve cultures provide habitat for exotic tunicates in southern Brazil., Aquatic Invasions 4(1): 195-205
da Rocha, Rosana M.; Kremer, Laura P. (2005) Introduced ascidians in Paranagua Bay, Parana, southern Brazil., Revista Brasileira da Zoologia 22(4): 1170-1184
da Rocha, Rosana Moreira; Monniot, François (1995) Taxonomic and ecological notes on some Didemnum species (Ascidiacea, Didemnidae) from São Sebastião Channel, south-eastern Brazil, Revista Brasiliera de Biologia 55(4, Part 1): 639-649
Dalby, James E. Jr.; Young, Craig M. (1992) Role of early post-settlement mortality in setting the upper depth limit of ascidians in Florida epifaunal communities, Marine Ecology Progress Series 80: 221-228,
David, Gwendolyn K.; Marshall, Dustin J.; Riginos, Cynthia (2010) Latitudinal variability in spatial genetic structure in the invasive ascidian, Styela plicata, Marine Biology 157: 1955-1965
David, Matej; Gollasch, Stephan; Cabrini, Marina; Perkovic, Marko; Bosnjak, Dean; Virgilio, Damiano (2007) Results from the first ballast water sampling study in the Mediterranean Sea: the Port of Koper study., Marine Pollution Bulletin 54: 53-65
de Barros, Rodolfo C.; da Rocha, Rosana M.; Pie, Marcio R. (2009) Human-mediated global dispersion of Styela plicata. (Tunicata, Ascidiacea), Aquatic Invasions 4(1): 45-57
de Barros, Rodolfo Corrêa; da Rocha, Rosana Moreira (2021) Two new species of Styela (Tunicata: Ascidiacea) from the tropical West Atlantic Ocean, Zootaxa 4948: 276-286
de Rivera, Catherine, and 27 authors (2005) Broad-scale non-indigenous species monitoring along the West Coast in National Marine Sanctuaries and National Estuarine Research Reserves report to National Fish and Wildlife Foundation, National Fish and Wildlife Foundation, Washington, D.C.. Pp. <missing location>
Dias, G. M.; Rocha, R. M.; Lotufo, T. M. C.; Kremer, L. P. (2013) Fifty years of ascidian biodiversity research in Sao Sebastiao, Brazil, Journal of the Marine Biological Association of the United Kingdom 93(1): 273-282
El Nagar, Aliya; Huys, Rony; Bishop, John D. D. (2010) Widespread occurrence of the Southern Hemisphere ascidian Corella eumyota Traustedt, 1882 on the Atlantic coast of Iberia, Aquatic Invasions 5(2): 169-173
Emara, Ahmed; Belal, Aisha (2004) Marine fouling in Suez Canal, Egypt, Egyptian Journal of Aquatic Research 30A: 189-206
Espla, A. A. Ramos; Buencuerpo, V.; Vazquez, E.; Lafargue, F. (1992) Some biogeographical remarks about the ascidian littoral fauna of the straits of Gibraltar (Iberian sector), Bulletin de l'Institut Océanographique 9 (special): 125-131
Fairey, Russell; Dunn, Roslyn; Sigala, Marco; Oliver, John (2002) Introduced aquatic species in California's coastal waters: Final Report, California Department of Fish and Game, Sacramento. Pp. <missing location>
Fischer, W.; Schneider, M.; Bauchot, M.-L. (1987) <missing title>, FAO-CEE, Rome. Pp. <missing location>
Fortic, Ana ; Mavric; Borut; Pitacco; Valentina; Lipej, Lovrenc (2021) Temporal changes of a fouling community: Colonization patterns of the benthic epifauna in the shallow northern Adriatic Sea, Regional Studies in Marine Science 45(101818): Published online
Fortunato, Humberto F.M.; Lôbo-Hajdu, Gisele (2021) Quantification of the non-indigenous ophiuroid Ophiothela mirabilis Verrill, 1867 associated with marine sponges with different morphologies, Biological Invasions 16: 77=-93
Glasby, T. M. (1999) Effects of shading on subtidal epibiotic assemblages, Journal of Experimental Marine Biology and Ecology 234: 275-290
Goodbody, Ivan (2004) Diversity and distribution of ascidians (Tunicata) at Twin Cays, Belize, Atoll Research Bulletin 524: 1-22
Goodbody, Ivan; Webber, Mona (2003) The ascidian fauna of Port Royal, Jamaica I. Harbor and mangrove-dwelling species., Bulletin of Marine Science 73(2): 457-476
Granthom-Costa, Luciana Vieira; Werner Ferreira, Carlos Gustavo; Dias, Gustavo Muniz (2016) Biodiversity of ascidians in a heterogeneous bay from southeastern Brazil, Management of Biological Invasions 7: 5-12
Holman, Luke E.; Parker-Nance, Shirley; de Bruyn, Mark; Creer, Simon; Carvalho, Gary; Rius, Marc (2021) Managing human-mediated range shifts: understanding spatial, temporal and genetic variation in marine non-native species, Philosophical Transactions of the Royal Society of London B 377: 20210025
Huang, Zongguo (Ed.), Junda Lin (Translator) (2001) Marine Species and Their Distributions in China's Seas, Krieger, Malabar, FL. Pp. <missing location>
Ignacio, Barbara L.; Julio, Luciana M.; Junqueira, Andrea O. R; Ferreira-Silva, Maria A. G. (2010) Bioinvasion in a Brazilian Bay: filling gaps in the knowledge of southwestern Atlantic biota, None 5(9): <missing location>
Inglis, Graeme and 6 authors (2006a) Port of Lyttelton: Baseline survey for non-indigenous marine species, Biosecurity New Zealand Technical Paper 2005/01: 1-64
Inglis, Graeme and 5 authors (2006b) Port of Taranaki: Baseline survey for non-indigenous marine species, Biosecurity New Zealand Technical Paper 2005/04: 1-47
Inglis, Graeme and 6 authors (2006c) Port of Nelson: Baseline survey for non-indigenous marine species, Biosecurity New Zealand Technical Paper 2005/02: 1-43
Inglis, Graeme; Gust, Nick; Fitridge, Isla ; Floerl, Oliver; Woods, Chris. Barbara Hayden, Graham Fenwick (2005c) Gulf Harbour Marina: Baseline survey for non-indigenous marine species., Biosecurity New Zealand Technical Paper 2005(12): 1-39
Johnston, Emma L.; Keough, Michael J. (2002) Direct and indirect effects of repeated pollution events on marine hard-substrate assemblages, Ecological Applications 12(4): 1212-1228
Kaplan, Eugene H. (1988) A Field Gude to Southeastern and Caribbean Seashores, In: (Eds.) . , Boston. Pp. <missing location>
Karlson, Ronald H.; Osman, Richard W (2012) Species composition and geographic distribution of invertebrates in fouling communities along the east coast of the USA: a regional perspective, Marine Ecology Progress Series 458: 255–268
Keough, M. J. ; Ross, J. (1999) Introduced fouling species in Port Phillip Bay., In: Hewitt, C. L.; Campbell; M.;Thresher, R.; Martin,(Eds.) Marine Biological Invasions of Port Phillip Bay, Victoria. , Hobart, Tasmania. Pp. 193-225
Kestrup, Asa M.; Ricciardi, Anthony (2009a) Environmental heterogeneity limits the local dominance of an invasive freshwater crustacean, Biological Invasions 11: 2095-2105
Kott, P. (1998) Tunicata, Zoological Catalogue of Australia 34: 51-252
Kott, Patricia (1985) The Australian Ascidiacea Part 1, Phlebobranchia and Stolidobranchia., Memoirs of the Queensland Museum 23: 1-440
Koukouras, Athanasios; Voultisiado-Koukoura, Eleni; Kevrekidis, Theodoros; Vafidis, Dimitri (1995) Ascidian fauna of the Aegean Sea with a checklist of the Mediterranean and Black Sea species, Annales de l Institut Oceanographique, Paris 71(1): 19-34
Lambert, C. C.; Lambert, G. (1998) Non-indigenous ascidians in southern California harbors and marinas., Marine Biology 130: 675-688
Lambert, Charles C; Lambert, Gretchen (2003) Persistence and differential distribution of nonindigenous ascidians in harbors of the Southern California Bight., Marine Ecology Progress Series 259: 145-161
Lambert, Gretchen; Faulkes, Zen; Lambert, Charles C.; Scofield, Virginia L. (2005) Ascidians of South Padre Island, Texas, with a key to species., Texas Journal of Science 57(3): 251-262
Lambert, Gretchen; Karney, Richard C.; Rhee, Walter Y.; Carman, Mary R. (2016) Wild and cultured edible tunicates: a review, Management of Biological Invasions 7: 59-66
Lesueur, C. A. (1823) Descriptions of several new species of Ascidia, Journal of the Academy of Natural Sciences of Philadelphia 3: 2-8
Li, Xinzhengl Shenz. Anglv; Xu, zhaou (2008) Report on some shrimos from dontou Island, Zhejiang, China (Decapoda, Natantia, Crustaceana (Leiden) <missing volume>: <missing location>
Locke, Andrea (2009) A screening procedure for potential tunicate invaders of Atlantic Canada., Aquatic Invasions 4(1): 71-79
Lomonaco, Cecilia; Santos, Andre S.; Christoffersen, Martin l. (2011) Effects of local hydrodynamic regime on the individual’s size in intertidal Sabellaria (Annelida: Polychaeta: Sabellariidae) and associated fauna at Cabo Branco beach, north-east Brazil, Marine Biodiversity Records 4(e76): Published online
doi:10.1017/S1755267211000807;
Lopes, Rubens M. (Ed.) (2009) <missing title>, Ministry of the Environment, Brasilia, Brazil. Pp. 1-440
Lopez-Legentil, Susanna; Legentil, Miquel L.; Erwin, Patrick M.; Turon, Xavier (2015) Harbor networks as introduction gateways: contrasting distribution patterns of native and introduced ascidians, Biological Invasions 17: 1623-1638
DOI 10.1007/s10530-014-0821-z
Lord, Joshua P.; Calini, Jeremy M.; Whitlatch, Robert B. (2015) Influence of seawater temperature and shipping on the spread and establishment of marine fouling species, Marine Biology 162: 2481-2492
Low-Pfeng, Antonio; Recagno, Edward M. Peters (2012) <missing title>, Geomare, A. C., INESEMARNAT, Mexico. Pp. 236
Manoudis, Georgios; Antoniadou, Chryssanthi; Dounas, Konstantinos; Chintiroglou, Chariton Ch. (2005) Successional stages of experimental artificial reefs deployed in Vistonikos gulf (N. Aegean Sea, Greece) : Preliminary results, Belgian Journal of Zoology 135(2): 209-215
Marins, Flavia O.; Novaes, Roberto L. M.; Rocha, Rosana M.; Junquiera, Andrea O. R. (2010) Non indigenous ascidians in port and natural environments in a tropical Brazilian bay, Zoologia 27(2): 213-222
Marshall, J. I.; Rowe, F. W. E.; Fisher; Smith, D. F. (1980) Alterations to the relative species-abundance of ascidians and barnacles in a fouling community due to screens, Australian Journal of Marine and Freshwater Research 31: <missing location>
Mastrototaro, F.; D’Onghia, G.; Tursi, A. (2008) Spatial and seasonal distribution of ascidians in a semi-enclosed basin of the Mediterranean Sea., Journal of the Marine Biological Association of the United Kingdom 88(5): 1053-1061
Mead, A.; Carlton, J. T.; Griffiths, C. L. Rius, M. (2011b) Introduced and cryptogenic marine and estuarine species of South Africa, Journal of Natural History 39-40: 2463-2524
Millar, R. H. (1958) Some ascidians from Brazil., Annals and Magazine of Natural History 13(1): 497-514
Monniot, C.; Monniot, F. (1985) [Littoral ascidians of Guadeloupe Island: IX. Characteristics of populations, ecology, relationships with the world fauna] (French), Tethys 11(3-4): 203-213
Monniot, Claude (1972) [Stolidobranch Ascidians of Bermuda[ (French), Bulletin du Museum National d'Histoire Naturelle. 4e Serie. Section A. Zoologie, Biologie et Ecologie Animales 43: 617-643
Monniot, Claude (2002) Stolidobranch ascidians from the tropical western Indian Ocean., Zoological Journal of the Linnean Society 135: 65-120
Monniot, Claude; Monniot, Francoise (1994) Additions to the inventory of Eastern tropical Atlantic Ascidians: arrival of cosmopolitan species., Bulletin of Marine Science 54(1): 71-93
Monniot, Claude; Monniot, Francoise; Griffiths, Charles; Schleyer, Michael (2001) South African Ascidians., Annals of the South African Museum 108(1): 1-141
Monniot, Francoise; Monniot, Claude (1997) Ascidians collected in Tanzania, Journal of East African Natural History 86: 1-35
Mook, David (1983) Indian River fouling organisms, a review, Florida Scientist 26(3/4): 162-167
Moura, Carlos J.; Collins, Allen G.; Santos, Ricardo S.; Lessios, Harilaos (2019) Predominant east to west colonizations across major oceanic barriers: Insights into the phylogeographic history of the hydroid superfamily Plumularioidea, suggested by a mitochondrial DNA barcoding marker, Ecology and Evolution 9: :13001–13016.
DOI: 10.1002/ece3.5608
Naranjo, S. A.; Carballo, J. C.; Garcia-Gomez, J. C. (1996) Effects of environmental stress on ascidian populations in Algeciras Bay (southern Spain)., Marine Ecology Progress Series 144: 119-131
Nichols, Claire L.; Lambert, Gretchen; Nydam, Marie L. (2023) Continued persistence of non-native ascidians in Southern California harbors and marinas, Aquatic Invasions 18(1): 1-22
https://doi.org/10.3391/ ai.2023.18.1.101962
Nishikawa, T. (1991) The ascidians of the Japan Sea. II., Publications of the Seto Marine Biological Laboratory 35: 25-170
O' Beirn, F. X.; Ross, P. G.; Luckenbach, M.W. (2004) Organisms associated with oysters cultured in floating systems in Virginia., Journal of Shellfish Research 23(3): 825-829
Orensanz, Jose Maria and 14 other authors (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic, Biological Invasions 4(1-2): 115-143
Pestana, L. B.; Dias, G. M.; Marques, A. C . (2021) Spatial and temporal diversity of non-native biofouling species associated with marinas in two Angolan bays, African Journal of Marine Science 42(4): 413-422
Pestana, Lueji Barros; Dias, Gustavo Muniz; Marquesa, Antonio Carlos (2017) A century of introductions by coastal sessile marine invertebrates in Angola, South East Atlantic Ocean, Marine Pollution Bulletin 125: 426-a432
Pineda, M. Carmen; Lopez-Legentil, Susanna; Turon, Xavier (2013) Year-round reproduction in a seasonal sea: biological cycle of the introduced ascidian Styela plicata in the Western Mediterranean, Marine Biology 160: 221-230
Pineda, M. Carmen; McQuaid, Christopher D.; Turon, Xavier; Lopez-Legentil, Susanna; Ordonez, Výctor; Rius, Marc (2012) Tough adults, frail babies: An analysis of stress sensitivity across early life-history stages of widely introduced marine invertebrates, None 7(10): e46672
Pineda, M. Carmen; Turon, Xavier; Pérez-Portela, Rocío; López-Legentil, Susanna (2016) Stable populations in unstable habitats: temporal genetic structure of the introduced ascidian Styela plicata in North Carolina, Marine Biology 163: Published online
Pineda, Mari Carmen; Lopez-Legentil, Susanna; Turon, Xavier (2011) The whereabouts of an ancient wanderer: global phylogeography of the solitary ascidian Styela plicata, None 6(9): e25495
Pineda, Mari Carmen; Turon, Xavier; López-Legentil, Susanna (2012) Stress levels over time in the introduced ascidian Styela plicata: the effects of temperature and salinity variations on hsp70 gene expression, Cell Stress and Chaperones <missing volume>: published online
Plough, Harold H. (1978) <missing title>, Johns Hopkins University Press, Baltimore. Pp. <missing location>
Pyo, Jooyeon; Lee, Taekjun; Shin, Sook (2012) Two newly recorded invasive alien ascidians (Chordata, Tunicata, Ascidiacea) based on morphological and molecular phylogenetic analysis in Korea, Zootaxa 3368: 211-228
Ramalhosa Patrício; Gestoso, Ignacio Rocha Rosana M.; Lambert, Gretchen; Canning-Clode, João (2021) Ascidian biodiversity in the shallow waters of the Madeira Archipelago: Fouling studies on artificial substrates and new records, Regional Studies in Marine Science 43(1901672): Published online
Reish, Donald J.; Kauwling, Thomas J.; Schreiber, Traynor C. (1975) Annotated checklist of the marine invertebrates of Anaheim Bay, California Department of Fish and Game Fish Bulletin 165: 41-51
Rho, Boon Jo (1995) The Ascidians (Tunicata) from Chindo Islands, Korea, Korean Journal of Systematic Zoology 11(1): 125-145
Rho, Boon Jo; Choe, Byung Lae; Song, Jun-Im; Lee Young Ja (2000) Ascidians of Tangsa and its adjacent waters in Korea strait., Korean Journal of Systematic Zoology 16(1): 39-53
Rho, Boon Jo; Lee, Ji-Eun (1991) A systematic study of the Ascidians in Korea, Korean Journal of Systematic Zoology 7(2): 195-220
Rho, Boon Jo; Park, Kyung-Sook (1998) Taxonomy of ascidians from Geojedo Island in Korea, Korean Journal of Systematic Zoology 14(3): 173-192
Rius, M.; Griffths, C. W. (2011) Alien & Invasive Animals: A South African Perspective, Random House Struik, Johannesburg, South Africa. Pp. 71-75
Rius, Marc and 7 authors (2014) Range expansions across ecoregions: interactions of climate change, physiology and genetic diversity, Diversity and Distributions 23: 76-88
Rius, Marc; Branch, George M.; Griffiths, Charles L.; Turon, Xavier (2010) Larval settlement behaviour in six gregarious ascidians in relation to adult distribution, Marine Ecology Progress Series 418: 151-163
Rius, Marc; Turon, Xavier; Marshall, Dustin J. (2009) Non-lethal effects of an invasive species in the marine environment: the importance of early life-history stages, Oecologia 159: 873-882
Rodriguez, Laura F.; Ibarra-Obando, Silvia E. (2008) Cover and colonization of commercial oyster (Crassostrea gigas) shells by fouling organisms in San Quintin Bay, Mexico, Journal of Shellfish Research 27(2): 337-343
Ruiz, Gregory M.; Geller, Jonathan (2018) Spatial and temporal analysis of marine invasions in California, Part II: Humboldt Bay, Marina del Re, Port Hueneme, and San Francisco Bay, Smithsonian Environmental Research Center & Moss Landing Laboratories, Edgewater MD, Moss Landing CA. Pp. <missing location>
Ruiz, Gregory; Geller, Jonathan (2021) Spatial and temporal analysis of marine invasions: supplemental studies to evaluate detection through quantitative and molecular methodologies, Marine Invasive Species Program, California Department of Fish and Wildlife, Sacramento CA. Pp. 153 ppl.
Simkanin, Christina; Fofonoff, Paul W.; Larson, Kriste; Lambert, Gretchen; Dijkstra, Jennifer A.; Ruiz, Gregory M. (2016) Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska, Marine Biology 163: Published online
Sims, Linda L. (1984) Osmoregulatory capabilities of three macrosympatric stolidobranch ascidians, Styela clava Herdman, Styela plicata Leseur, and Styela montereyensis (Dall)., Journal of Experimental Marine Biology and Ecology 82: 117-129
Skinner, Luís F.; Barboza, Danielle F.; Rocha, Rosana M. (2016) Rapid Assessment Survey of introduced ascidians in a region with many marinas in the southwest Atlantic Ocean, Brazil, Management of Biological Invasions 7(1): 13-20
Soors, Jan; Faasse, Marco; Stevens, Maarten; Verbessem, Ingrid; De Regge, Nico;Van den Bergh, Ericia (2010) New crustacean invaders in the Schelde estuary (Belgium), Belgian Journal of Zoology 140: 3-10
Streit, Olivia T; Lambert, Gretchen; Erwin, Patrick M.; Lopez-Legentil, Susanna (2021) Diversity and abundance of native and non-native ascidians in Puerto Rican harbors and marinas, Marine Pollution Bulletin 167(112262): Published online
Sutherland, John P.; Karlson, Ronald H. (1977) Development and stability of the fouling community at Beaufort, North Carolina, Ecological Monographs 47: 425-446
Thiyagarajan, Vengatesen; Qian, Pei-Yuan (2003) Effect of temperature, salinity and delayed attachment on development of the solitary ascidian Styela plicata (Lesueur)., Journal of Experimental Marine Biology and Ecology 290: 133-146
Tracy, Brianna M.; Reyns, Nathalie B. (2014) Spatial and temporal patterns of native and invasive ascidian assemblages in a Southern California embayment, Aquatic Invasions 9: In press
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/
Ulman, Aylin and 17 authors (2017) A massive update of non-indigenous species records in Mediterranean marinas, PeerJ 5( e3954): <missing location>
Valero-Jimenez, Claudio A.; Perez-Portela, Rocio; Lopez-Legentil, Susanna (2012) Characterization of novel microsatellite markers from the worldwide invasive ascidian Styela plicata, Conservation Genetics Resources 4: 559-561
Van Name, Willard G. (1912) Simple ascidians of the coasts of New England and neighboring British provinces., Proceedings of the Boston Society of Natural History 34: 439-619
Van Name, Willard G. (1921) Ascidians of the West Indian region and southeastern United States., Bulletin of the American Museum of Natural History 44: 283-494
Van Name, Willard G. (1945) The North and South American ascidians, Bulletin of the American Museum of Natural History 84: 1-462
Villalobos, Stephanie M.; Lambert, Gretchen; Shenkar, Noa; López-Legentil, Susanna (2017) Distribution and population dynamics of key ascidians in North Carolina harbors and marinas, Aquatic Invasions 12(4): 447-458
http://www.aquaticinvasions.net/2017/AI_2017_Villalobos_etal.pdf
Weiss, Charles M. (1948) The seasonal occurrence of sedentary marine organisms in Biscayne Bay, Florida., Ecology 29(2): 153-172
Whitten, H. L.; Rosene, Hilda F.; Hedgpeth, J. W. (1950) Invertebrate fauna of Texas Coast Jetties: a preliminary survey., Publications in Marine Science of the University of Texas 1(2): 53-87
Wiltshire, K.; Rowling, K.; Deveney, M. (2010) <missing title>, South Australian Research and Development Institute, Adelaide. Pp. 1-232
Wyatt, Alex S. J.; Hewitt, Chad L.; Walker, Di I.; Ward, Trevor J. (2005) Marine introductions in the Shark Bay world heritage property, Western Australia: a preliminary assessment., Diversity and Distributions 11: 33-44