Invasion History
First Non-native North American Tidal Record: 2013First Non-native West Coast Tidal Record:
First Non-native East/Gulf Coast Tidal Record: 2013
General Invasion History:
The encrusting bryozoan Juxtacribrilina mutabilis was described from the Akkeshi-ko estuary, northern Japan in 2014, but was found at about the same time off Kristineberg on the west coast of Sweden (Ostrovsky, cited by Ito et al. 2015), and off Norway (Trott and Enterline 2019). It is probably native to the Northwest Pacific, including Japan and possibly Russia (Ito et al. 2015). In the Akkeshi-ko estuary, C. mutabilis was exclusively associated with eelgrass (Zostera marina) (Ito et al. 2015), but in Sweden was associated with brown algae (Fucus spp. (Rockweed), Laminaria sp. (Kelp) (Ostrovsky, cited by Ito et al. 2015). This bryozoan was once found on a hard substrate, as a dead colony on a plastic bin associated with tsunami debris (McCuller et al. 2018). A possibly conspecific form, Klugerella aragoi ex grege has been reported from the Russian coasts of the Seas of Japan and Okhotsk (Kubanin, 1998, cited by Ito et al. 2015). The term ‘ex grege’ means ‘one of the group’, meaning that it is related to K. aragoi, known from Florida and Egypt (Bock 2019). Unlike C. mutabilis in Japan, K. aragoi ex grege in Pacific Russia does not have multiple types of zooids, and settles on hard substrates (shells, wharves, fouling plates, boats) as well as eelgrass and algae (Kubanin, 1998, cited by Ito et al. 2015). These differences could be related to local conditions or indicate genetic divergence. In 2018, J. mutabilis was found growing on eelgrass in Casco Bay, Maine its first occurrence in the Northwest Atlantic (Trott and Enterline 2019). Recent opportunistic detections have found J. mutabilis throughout the Atlantic and Gulf of St. Lawrence coasts of Nova Scotia, Canada and other locations in the Canadian Maritime Provinces and the east coast of Newfoundland, on various substrates such as eelgrass, macroalgae, plastic settlement plates, and rocks (Pratt et al., in prep).
North American Invasion History:
Invasion History on the East Coast:
On September 12, 2018, large numbers of colonies of C. mutabilis were found on eelgrass growing around three islands—Clapboard, Mackworth, and Hog Islands in Casco Bay, Maine. Most colonies were on the brown, decaying tips of the leaves. All three zooid types were found, but the I (intermediate) form was most common. Ancestrulae and young colonies were found on eelgrass. No colonies were found on shell, cobble, or bedrock. At one location, colonies were also found on decaying, drifting kelp. Colonies of C. mutabilis were found near Portland Harbor, a major seaport, receiving ships from Europe. However, more surveys are needed to determine whether other populations are found on the East Coast of North America (Trott and Enterline 2019). Recent opportunistic detections have found J. mutabilis throughout the Atlantic and Gulf of St. Lawrence coasts of Nova Scotia, Canada, on the Gulf of St Lawrence coast of New Brunswick, at numerous locations throughout Prince Edward Island, throughout the Magdalen Islands and on the northeast coast of Newfoundland (Pratt et al., in prep). The species has been found on a variety of substrates such as eelgrass, macroalgae, plastic settlement plates, and rocks throughout the region.
Invasion History Elsewhere in the World:
The encrusting bryozoan Juxtacribrilina mutabilis was described from the Akkeshi-ko estuary, northern Japan in 2014, but was found in 2008 in Bergen, Norway, in 2011, off Kristineberg on the west coast of Sweden, and Portavadie, Scotland in 2013 (Dick et al. 2020). It is probably native to the Northwest Pacific, including northern Japan, China and Russia (Ito et al. 2015). Populations in the Akkeshi-ko estuary had a low genetic diversity, suggestive of a recent range expansion or introduction to Japan (Ito et al. 2015), but more information is needed on the occurrence, genetics, and systematics of this species in the Northwest Pacific. In the Akkeshi-ko estuary, J. mutabilis was exclusively associated with eelgrass (Zostera marina) (Ito et al. 2015), but in Sweden was associated with eelgrass (Zostera spp.) and brown algae (Fucus spp), rockweed (Cytoseira tamariscifolia), and kelp (Laminaria spp.) (Ostrovsky, cited by Ito et al. 2015). In Scotland, J. mutabilis was found on fouling panels suspended from a marina's pontoons, and on algae attached to the pontoon. In Pacific Russia, J. mutabilis (as Membraniporella aragoni) was found attached to marinas, ship hulls, and fouling panels (Kubanin 1977, cited by Dick et al. 2020).This bryozoan was found once in the Northeast Pacific, on a hard substrate, as a single dead colony on a shell of Mytilus galloprovincialis.
Historical and genetic data suggest a northwest Pacific origin of Juxtacribrilina mutabilis, probably in Pacific Russia, where it was identified as Membraniporella aragoi, but with a possible recent range expansion to northern Japan, where it was described in 2014 (Ito et al. 2015; Trott et al. 2019; Dick et al. 2020). Atlantic populations were detected in Norway in 2008, the west coast of Sweden in 2011, Scotland in 2013, and Casco Bay, Maine, in 2018 (Ito et al. 2015; Trott and Enterline 2018; Dick et al. 2020). Atlantic (Maine, Sweden, Norway) populations show some genetic divergence of from Japanese haplotypes, but not necessarily inconsistent with a recent introduction to the Atlantic. The expansion of trans-Arctic shipping, as Arctic ice declines due to global warming (Miller and Ruiz 2014), is likely vector for the spread of this bryozoan to the Atlantic (Dick et al. 2020). In a recent survey, Juxtacribrilina mutabilis was found to have a very wide range in Europe, from Svalbard and Norway to Spain, Finland, in the Baltic Sea, and Crete (Greece) in the Mediterranean, thus indicating a very wide range of temperature and salinity tolerance (Martaeng et al. 2023).
Description
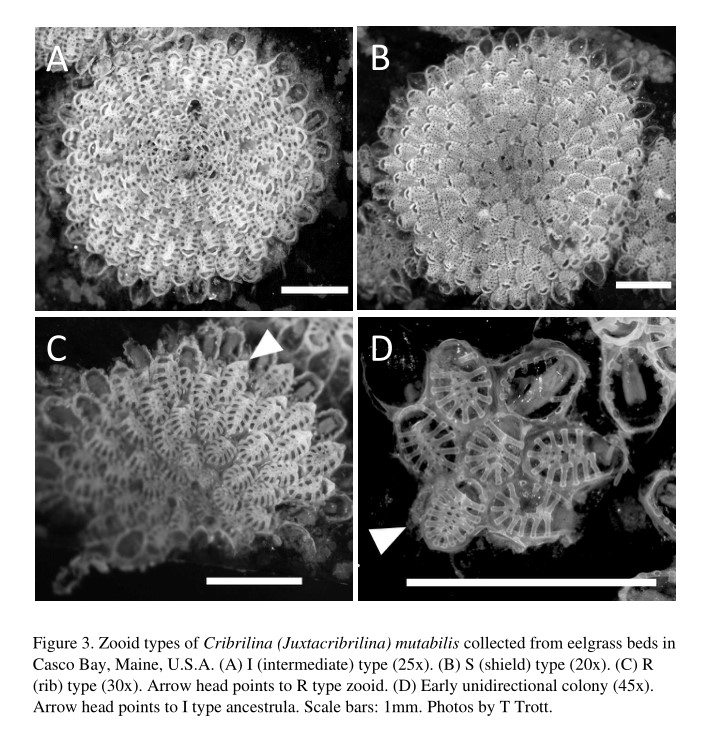
The encrusting bryozoan Juxtacribrilina mutabilis forms small (5–7mm), flat, roughly circular, whitish to tan colonies with orange embryos. This species is polymorphic with three types of zooids, each varying in abundance seasonally (Ito et al. 2015; Trott and Enterline 2019). These types are so distinct morphologically, they might be misidentified as different species. However, DNA sequencing of the mitochondrial COI gene shows they are from the same species (Ito et al. 2015). In general, the zooids are oval, separated by a sharp groove, have 2 to 3 distal spines, and lack avicularia. The gymnocyst is smooth proximally and barely distinguishable laterally. The highly convex spinocyst has large, non-articulated costae (ribs), their width and degree of fusion distinguishing the zooid types. An opesia occupies the entire frontal area of a zooid and is bounded by a raised mural lip. Reproductive zooids have a vestigial ooecium (ovicell) at their distal end and contain a single embryo (Ito et al. 2015; Trott and Enterline 2019). The common name "Ribbed Bryozoan" has recently been given to Juxtacribrilina mutabilis for the species based on its distinctive rib-like costae around the zooid margins (Conrad Pratt, personal correspondence).
The ancestrula (initial zooid of a colony) can be any of the three types: R (rib), I (intermediate), and S (shield). The ancestrula is usually surrounded by seven periancestrular zooids, one distal and two each in distolateral, proximolateral, and proximal positions. Initially, budding is in the distal direction and results in a unidirectional colony. Growth in proximal and proximolateral directions follow producing a more circular colony (Ito et al. 2015; Trott and Enterline 2019).
The three zooid types differ most notably in the number and development of the costal ribs. R-type zooids have the likeness of a human ribcage formed by 8–12 strongly developed widely spaced ribs, fused along the midline that has no intercostal pores. I-type zooids are similar in shape to R-type ones but have 8–13 ribs as wide as the width of the intercostal openings. The ribs are fused along the midline creating a narrow elliptical shield with scattered pores. S-type zooids have 12–15 ribs fused along their lateral margins creating a broad shield with many small intercostal pores arranged in radial series. At least two zooid types can co-occur in a colony, however no colonies containing a mix of R- and S-types have been observed (Ito et al. 2015; Trott and Enterline 2019).
Colonies of Juxtacribrilina mutabilis in the Akkeshi-Ko estuary grew exclusively on eelgrass (Zostera marina) (Ito et al. 2015). In Casco Bay, Maine, most specimens were found on live eelgrass blades or their decaying tips, but some were found on young Knotted Wrack (Ascophyllum nodosum) and decaying laminarian kelp (Trott and Enterline 2019). Off Kristineberg, Sweden, J. mutabilis grew on Zostera sp., Laminaria spp., and Fucus spp. (Ostrovsky, cited Ito et al. 2015). One dead colony of this bryozoan was found on a mussel shell attached to tsunami debris washed onto the US North Pacific coast (McCuller and Carlton 2018). Reports from Pacific Russia of bryozoans identified as Klugerella sp. aff. aragoi, possibly J. mutabilis instead, describe settlement on shells, cobbles, pilings, and boats (Kubanin 1997 cited in Ito et al. 2015).
Colonies on eelgrass in Japan, Russia, Sweden, Norway, and Maine show broadly similar patterns of seasonally changing morphology, but zooid frequencies vary on non-eelgrass substrates. Zooids encircling thin stalks of the brown alga Cystoseira tamariscifolia, in Sweden, become greatly elongated (Dick et al. 2020).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Bryozoa | |
| Class: | Gymnolaemata | |
| Order: | Cheilostomata | |
| Family: | Cribrilinidae | |
| Genus: | Juxtocribrilina | |
| Species: | mutabilis |
Synonyms
Potentially Misidentified Species
Native, amphi-Atlantic (Trott and Enterline 2019)
Cribrilina cryptooecium
Native, amphi-Atlantic (Trott and Enterline 2019)
Cribrilina macropunctata
Native, west Atlantic (Trott and Enterline 2019)
Ecology
General:
The encrusting bryozoan Juxtacribrilina mutabilis forms small colonies on eelgrass (Zostera marina). Juxtacribrilina annulata, lecithotrophic larvae are brooded and released, settling after a short planktonic period. Chances of settling on favorable substrates are increased by behavioral responses to metabolites liberated from algae and other fouling organisms, in addition to the influence of physical factors on settlement (Yagunova and Ostrovsky 2010). The settled larva becomes an ancestrula. In J. mutabilis, the ancestrula can be any of the three zooid types. Zooids do not transform from one kind to another but can bud the same or an alternative type. The overwintering type is not known, but the late summer appearance of nonreproductive S-type zooids suggests this one may be, at least in Japan. Since S-type zooids do not reproduce sexually, Ito et al. (2015) hypothesize that resources otherwise invested into reproduction might instead be used in the development of the stronger, more complete, S-type costal shield that may offer better protection from stormy winter conditions.
Juxtacribrilina mutabilis is known only from cold-temperate climates in the northern hemisphere, specifically northern Japan, Norway, the west coast of Sweden, and Casco Bay, Maine. Its type locality, the Akkeshi-ko estuary, has salinities ranging from 6 to 27 PSU, but J. mutabilis was found in a narrower range of 24.5 to 27.6 PSU. Among reported locations, its preferred habitats seem to be in the poly- and euhaline range (21-35 PSU) (Ito et al. 2015; Trott and Enterline 2019). In the Akkeshi-ko estuary, colonies of C. mutabilis are known to occur only on Eelgrass (Zostera marina). In Sweden and Casco Bay, they occur mainly on eelgrass and brown algae (Saccharna latissima; Cytoseira tamariscifolia; Fucus spp.; Ascophyllum nodosum) (Ito et al. 2015; Trott and Enterline 2019). This bryozoan is often found on dying tips of living eelgrass blades and sometimes on decaying drift algae, but this may be because the colonies outlive the plants (Trott and Enterline 2019). In a marina in Scotland, J.mutabilis was found on fouling plates suspended from pontoons. One colony was found on unidentified algae growing on a pontoon (Dick et al. 2020). As bryozoans, colonies of J. mutabilis are suspension feeders, feeding on phytoplankton and detritus.
Food:
Phytoplankton, detritus
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Grass Bed | None |
| General Habitat | Rocky | None |
| General Habitat | Oyster Reef | None |
Life History
The encrusting bryozoan Juxtacribrilina mutabilis forms small colonies on Eelgrass (Zostera marina)). Juxtacribrilina annulata, lecithotrophic larvae are brooded and released, settling after a short planktonic period. Chances of settling on favorable substrates are increased by behavioral responses to metabolites liberated from algae and other fouling organisms, in addition to the influence of physical factors on settlement (Yagunova and Ostrovsky 2010). The settled larva becomes an ancestrula. In J. mutabilis, the ancestrula can be any of the three zooid types. Zooids do not transform from one kind to another but can bud the same or an alternative type. The overwintering type is not known, but the late summer appearance of nonreproductive S-type zooids suggests this one may be, at least in Japan. Since S-type zooids do not reproduce sexually, Ito et al. (2015) hypothesize that resources otherwise invested into reproduction might instead be used in the development of the stronger, more complete, S-type costal shield that may offer better protection from stormy winter conditions.
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 4 | Field, Bergen, Norway (Dick et al. 2020) |
| Maximum Temperature (ºC) | 18 | Field, Casco Bay, Maine (Dick et al. 2020) |
| Minimum Salinity (‰) | 21 | Field, Kristineberg Marine Station, Sweden (Trott and Enterline 2018) |
| Maximum Salinity (‰) | 35 | Field, Casco Bay. ME (Trott and Enterline 2019). |
| Minimum Length (mm) | 5 | Typical colony size (Ito et al. 2015) |
| Maximum Length (mm) | 7 | Typical colony size (Ito et al. 2015) |
| Broad Temperature Range | None | Cold-Temperate |
| Broad Salinity Range | None | Polyhaline-Euhaline |
General Impacts
Ecological and economic impacts of the encrusting bryozoan, Juxtacribrilina mutabilis, are unknown. However, it has been found growing on Eelgrass (Zostera marina). An abundant fouling organism could adversely affect the growth and survival of Eelgrass, which an important component of marine habitats, and an indicator of water and habitat quality. The occurrence of this bryozoan on Eelgrass should be monitored.
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Bock, Phillip 2003-2013 Recent and Fossil Bryozoa. <missing URL>Dick, Matthew H.; Waeschenbach, Andrea; Trott, Thomas J.; Onishi, Takumi; Beveridge, Chris ; Bishop, John D. D.; Ito, Minako; Ostrovsky, Andrew N. (2020) Global distribution and variation of the Invasive cheilostome bryozoan Cribrilina mutabilis, Zoological Science 37: 485-497
Ito, K.; Goshima, S.; Nakao, S. (1996) Growth and reproduction of the generalist opisthobranch Haloa japonica: effect of algal seasonality on growth rate, Marine Biology 126: 395-401
Martaeng. Rasmus Obst, Matthias; Kuklinski, iotr (2023) Phylogeographic study using autonomous reef monitoring structures indicates fast range expansion of the invasive bryozoan Juxtacribrilina mutabilis, Hydrobiologia <missing volume>: Published online
https://doi.org/10.1007/s10750-023-05184-9
Trott, Thomas J.; Enterline, Claire (2019) First record of the encrusting bryozoan Cribrilina ( Juxtacribrilina ) mutabilis (Ito, Onishi and Dick, 2015) in the Northwest Atlantic Ocean, BioInvasions Records 8: 598-607
Yagunova, Ekaterina B.; Ostrovsky, Andrew N. (2010) The influence of substrate type on sexual reproduction of the bryozoan Cribrilina annulata (Gymnolaemata, Cheilostomata): A case study from Arctic seas, Marine Biology Research 6: 263-270