Invasion History
First Non-native Panama (Pacific) Tidal Record: 2008First Non-native Panama (Caribbean) Tidal Record: 2004
Panama Invasion History:
Invasion history elsewhere in the world:
In later SERC sampling, Hippoporina indica was found in Belize in 2005, and in marine waters at the Caribbean and Pacific ends of the Panama Canal (2004 and 2008, respectively) (Ruiz et al., unpublished data).
Description
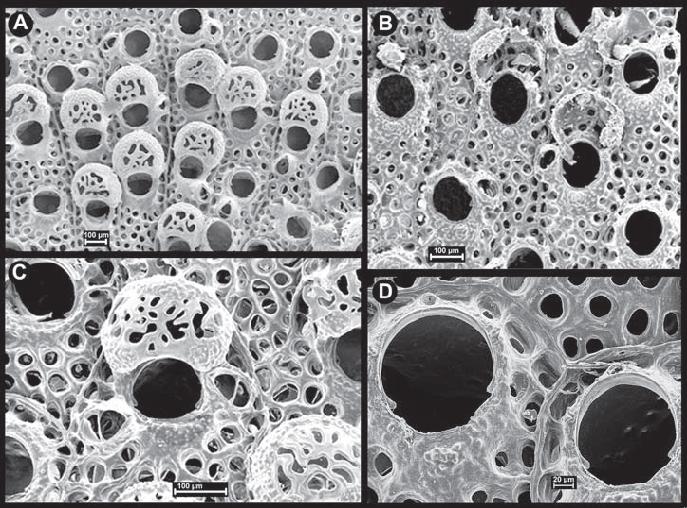
Hippoporina indica forms encrusting colonies. The zooids are short, rectangular, and variable in size (mean zooid length = 0.37 mm, mean zooid width = 0.27 mm, N=65; McCann et al. 2007). The frontal surfaces of the zooids have large marginal and frontal pores except for a granular unperforated central portion of frontal wall adjacent to the orifice. Up to three spines occur above the orifice; they are usually lost early in colony development, but the spine bases sometimes remain visible. The orifice is large relative to the zooid size. It is hoof-shaped, with a sub-circular anterior region and a shallowly convex proximal region. The two regions are separated by triangular, proximally slanting, hinging denticles. The granular calcification below the orifice is raised into a low peristome with a central peak. The orifice sometimes has two lateral processes on the rim. On one or both sides of the orifice, there are large umbos supporting avicularia (some zooids may have up to three avicularia, many zooids have none). These are rounded proximally, with narrow crossbars and short, pointed, triangular mandibles, and oriented laterally or disto-laterally toward the orifice. Similar triangular avicularia may also occur on lateral margins or frontal surfaces of the zooids. The ovicell is hyperstomial (partially embedded in the distal zooid, but with an opening above the mother cell), with granular calcification and irregular pores (in size, shape, and spacing) covering most of its frontal surface (Description from McCann et al. 2007).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Bryozoa | |
| Class: | Gymnolaemata | |
| Order: | Cheilostomata | |
| Suborder: | Ascophora | |
| Family: | Hippoporinidae | |
| Genus: | Hippoporina | |
| Species: | indica |
Synonyms
Hippothyris hongkongensis (Liu et al., 2001)
Potentially Misidentified Species
Hippoporina americana Verrill USNM #648039 from the Gulf of Mexico, may or may not be the same species, but is probably not H. americana (McCann et al. 2007)
Ecology
General:
Life History- Hippoporina indica is an encrusting, calcified bryozoan colony composed of many individual zooids. The zooids feed by extending the ciliated tentacles of the lophophore as a funnel, creating a current, and driving food particles into their mouths. The food is guided along the tentacles and through the pharynx by the cilia. Larger food particles can be moved or captured by flicking or contracting the tentacles (Barnes 1983). Hippoporina indica belongs to a taxonomic group which has lecithotrophic larvae with a short planktonic period (less than 1 day, Hayward and Ryland 1998). Larvae settle on a substrate and metamorphose into the first zooid of a colony, an ancestrula (Hayward and Ryland 1998).
Ecology- In addition to PVC fouling plates, H. indica has been recorded from fishing rafts, fish cages, buoys, oysters, and barnacle shells in China (Lui and Li 1987, cited by McCann et al. 2007). It seems to be confined to waters of marine salinity (McCann et al. 2007).
Competitors:
Octocoral Stragulum bicolor
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Marinas & Docks | None |
| General Habitat | Vessel Hull | None |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
Tolerances and Life History Parameters
| Maximum Temperature (ºC) | 29.6 | Field, US East & West Coast marinas (Lord et al. 2015) |
| Age to Maturity | 0.1 | 22-25 days after settlement (Pillai and Madhavan1978) |
| Minimum Length (mm) | 11 | Colonies become reproductive at sizes between 11 and 20 mm. Maximum adult size- 100% of colonies exceeding 50 mm diameter are reproductive, but the maximum size of colonies was not given (Karande and Udhayakumar 1992). |
| Broad Temperature Range | None | Warm temperate-Tropical |
| Broad Salinity Range | None | Polyhaline-Euhaline |
General Impacts
Economic ImpactsShipping, fisheries- Hippoporina indica has been reported as a dominant fouling bryozoan in many areas of Hong Kong, found on fouling panels, ships, buoys, and in aquaculture facilities (Li, 1989).
Ecological Impacts
Competition- In India, its native range, Hippoporina indica grew more slowly than Arbopercula bengalensis, and Sinoflustra annae, two other bryozoans which have also been introduced to Southeast and Gulf Coast estuaries. Hippopornia indica persisted, however, owing to its greater longevity and ability to overgrow other species (Karande and Udhayakumar 1992).
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Barnes, D. K. A. (2002) Invasions by marine life on plastic debris., Nature 416: 808-809Calder, Dale R.; Faucci, Anuschka (2021) Shallow water hydroids (Cnidaria, Hydrozoa) from the 2002 NOWRAMP cruise to the Northwestern Hawaiian Islands, Zootaxa 5085: 1-73
Gordon, Dennis P. (2016) Bryozoa of the South China Sea: an overview, Raffles Bulletin of Zoology 34: 604-618
Gordon, Dennis P.; Hosie, Andrew M.; Carter, Michelle C. (2008) Post-2000 detection of warm-water alien bryozoan species in New Zealand- The significance of recreational vessels, Virginia Museum of Natural History Special Publication 15: 37-48
Hayward, P.J.; Ryland, J. S. (1998) Cheilostomatous Bryozoa. Part 1: Aeteoidea-Cribilinoidea., Synopses of the British Fauna 10 (2nd edition): 1-366
Karande, A. A.; Udhayakumar, M. (1992) Consequences of crowding on life-histories of cheilostome bryozoans in Bombay., Indian Journal of Marine Science 21: 133-136
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010) Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves, Journal of Shellfish Research 29(4): 975–984
https://doi.org/10.2983/0730-8000\(2007\)26[205:TIBDPA]2.0.CO;2
Kourkoutmani, Polyxeni; Michaloudi, Evangelia (2022) First record of the calanoid copepod Pseudodiaptomus marinus Sato, 1913 in the North Aegean Sea, in Thessaloniki Bay, Greece, None 11(BioInvasions Re): Published online
Li, C. Y. (1989) Collections of Papers on Marine Ecology in the Daya Bay, Third Institute of Oceanography (SOA) China Ocean Press, China. Pp. 106-111
Lindsay, Denise and 9 authors (2021) Genetic analysis of North American Phragmites australis guides management approaches, Aquatic Botany <missing volume>(1023589): Published online
https://doi.org/10.1016/j.aquabot.2022.103589
Liu, X. X.; Li, C. Y. (1987) Six new species of bryozoans from the waters of Hong Kong and Zhu Jiang Estuary., Journal of Oceanography in Taiwan Strait 6: 59-67
Liu, X. X.; Yin, X.; Ma, J. (2001) <missing title>, Science Press, Beijing. Pp. 860 p.
Lord, Joshua P.; Calini, Jeremy M.; Whitlatch, Robert B. (2015) Influence of seawater temperature and shipping on the spread and establishment of marine fouling species, Marine Biology 162: 2481-2492
Madhavan Pillai, S. R. (1978) A new species of Hippoporina (Ectoproccta, Ascophora) from Bombay coast., Current Science 47(2): 61-63
McCann, Linda D.; Hitchcock, Natasha Gray; Winston, Judith E.; Ruiz, Gregory M. (2007) Non-native bryozoans in coastal embayments of the southern United States: new records for the western Atlantic., Bulletin of Marine Science 80(2): 319-342
Oh, Dong-Ha and 6 auhtora (2021) Novel genome characteristics contribute to the invasiveness of Phragmites australis (common reed), Molecular Ecology Resources 31: 1142–1159.
DOI: 10.1111/mec.16293
Raveendran, T. V.; Wagh, A. B. (1993) Variations in biofouling on different species of Indian timbers., Mahasagar 26(1): 27-31
Schulte, David M. (2017) History of the Virginia Oyster Fishery, Chesapeake Bay, USA, Frontiers in Marine Science 4(127): Published online
doi: 10.3389/fmars.2017.00127
Srinivas, D.; Sawant, S. S.; Wagh, A. B. (1992) Biofoulers on aluminum and stainless steel panels at Vijaydurg harbour, central west coast of India, Indian Journal of Marine Science 41: 143-145
Teixeira, Larissa Marques Pires; Creed, Joel Christopher (2020) A decade on: an updated assessment of the status of marine non-indigenous species in Brazil, Aquatic Invasions 15: 30-43
Tilbrook, Kevin J. (2012) Bryozoa, Cheilostomata: First records of two invasive species in Australia and the northerly range extension for a third, Check List 8(1): 181-183
Udhayakumar, M.; Karande, A. A. (1989) Growth and breeding in cheilostome biofouling, Electra bengalensis in Bombay waters, west coast of India, Indian Journal of Marine Sciences 18: 95-99
Xavier, Everthon A; Aalmeida, na C.S.; Vieira, Leandro M. (2021) The role of artificial habitats on fouling bryozoan fauna in the southwestern Atlantic, Marine Pollution Bulletin 167: Published online
https://doi.org/10.1016/j.marpolbul.2021.112310