Invasion History
First Galapagos Record: 2016General Invasion History:
The colonial tunicate Symplegma rubra was first described from Bermuda (Monniot and Monniot 1972) but are widely distributed in the tropical West Atlantic and Indo-Pacific. It is considered cryptogenic in both of these area, including the Caribbean coast of Panama, but appears to be introduced in the tropical East Pacific.
Invasion History in the Galapagos:
In 2016, small colonies of S. rubra were collected at the Puerto Ayora Dock, on Santa Cruz Island, in the Galapagos Archipelago (Lambert 2019).
Invasion history elsewhere in the world:
Invasion history elsewhere in the world is not summarized for this species at this time.
Description
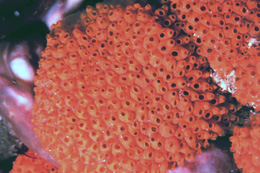
Symplegma rubra is a colonial tunicate growing in sheets about 30 cm long and 2–3 cm thick. The shared tunic is transparent. Zooids are oval, dorsoventrally compressed, 3–3.5 mm long and not arranged in systems. The apertures are oval. There are twelve curved oral tentacles, in two or three orders. The branchial sac has 14 rows of stigmata, with four longitudinal vessels on each side. The vessels on the left side are not parallel, with the most dorsal curving toward the dorsal lamina, ending at the ninth row of stigmata, while the second vessel ends at the tenth row, the prepharyngeal groove, and continues around the dorsal tubercle. The stomach has thirteen longitudinal folds. Symplegma rubra appears to alternate between male and female reproductions with ripe ovaries containing 8–9 oocytes, but undeveloped testes. During the male phase, the testis is large, with multiple lobes. The colonies are usually bright red to wine-colored, but can also be yellow, orange, or pink (Monniot and Monniot 1972; Monniot and Monniot 1997; da Rocha and Costa 2005).
Very small differences separate western Atlantic and eastern African specimens. In the latter, the zooid's stomach is slightly shorter, and the tadpoles develop in a pouch protruding from the side of the body wall. This brooding character, not observed in western Atlantic or Caribbean specimens, may be linked to the very widely spaced growth pattern of eastern African zooids, which were found living on flat bivalve shells (Monniot and Monniot 1997).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Chordata | |
| Subphylum: | Tunicata | |
| Class: | Ascidiacea | |
| Order: | Stolidobranchia | |
| Family: | Styelidae | |
| Genus: | Symplegma | |
| Species: | rubra |
Synonyms
Potentially Misidentified Species
Colors are variable, but rarely as bright red as S. rubra
Symplegma reptans
Colors are variable, but rarely as bright red as S. rubra
Ecology
General:
A colonial (or compound) tunicate consists of many zooids, bearing most or all of the organs of a solitary tunicate, but modified to varying degrees for colonial life. Colonial ascidians of the genera Symplegma have small, flattened, ovate zooids, but these are not arranged in systems. The zooids are embedded in a mass of tunic material. Each zooid has an oral and atrial siphon. Water is pumped into the oral siphon, through finely meshed ciliated gills on the pharynx, where phytoplankton and detritus is filtered, and passed on mucus strings to the stomach and intestines. Excess waste is expelled in the outgoing atrial water (Van Name 1945; Barnes 1983).
Colonial ascidians reproduce both asexually, by budding, and sexually, from fertilized eggs developing into larvae. Buds can form from the body wall of the zooid. Colonies vary in size and can range from small clusters of zooids to huge spreading masses. The zooids are hermaphroditic, with eggs and sperm being released to the atrial chamber. Eggs may be self-fertilized or fertilized by sperm from nearby animals, but some species have a partial block to self-fertilization. Eggs are brooded in the atrial chamber, and hatch into tadpole larvae, with a muscular tail and a notochord, eyespots, and a set of adhesive papillae. The lecithotrophic (non-feeding, yolk-dependent) larvae are expelled on hatching, and swim briefly before settlement. Swimming periods are usually less than a day, and some larvae can settle immediately after release, but the larval period can be longer at lower temperatures. On settlement, the tail is absorbed, the gill basket expands, and the tunicate begins to feed by filtering (Van Name 1945; Barnes 1983). Symplegma rubra is widespread in subtropical and tropical waters (da Rocha and Kremer 2005), and in habitats including coral reefs, mangroves, rocky shores, and marinas and docks (Goodbody, and Webber, 2003; da Rocha and Costa 2005).
Food:
Phytoplankton, detritus
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Marinas & Docks | None |
| General Habitat | Mangroves | None |
| General Habitat | Coral reef | None |
| General Habitat | Rocky | None |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
Life History
Tolerances and Life History Parameters
| Maximum Temperature (ºC) | 29.7 | Field, US East & West Coast marinas (Lord et al. 2015) |
| Minimum Salinity (‰) | 32 | Field, Capitania, Brazil (Marins et al. 2010) |
| Maximum Salinity (‰) | 24 | Field, Capitania, Brazil (Marins et al. 2010) |
General Impacts
Symplegma spp. are common fouling organisms on buoys, floats, pilings, and panels, but has not been reported to have significant economic impacts (Woods Hole Oceanographic Institution 1985). Symplegma spp. occurred on cultured mussels in Brazil but were not considered to have negative impacts on bivalve culture (Rocha 2009).
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Bullard, Stephan G.; Carman, Mary R.; Rocha, Rosana M.; Dijkstra, Jennifer A.; Goodwin, Anne M. (2011) Abundance and diversity of ascidians in the southern Gulf of Chiriquí, Pacific Panama, Aquatic Invasions 6(4): corrected proofCarman, Mary, and 8 authors (2011) Ascidians at the Pacific and Atlantic entrances to the Panama Canal, Aquatic Invasions 6(4): 371-380
da Rocha, Rosa Morales and 13 authors (2010) Inventory of ascidians (Tunicata, Ascidiacea) from the National Park La Restinga, Isla Margarita, Venezuela, Biota Neotropica 10: published online
da Rocha, Rosana M.; Costa, Luciana (2005) Ascidians (Urochordata: Ascidiacea) from Arriaal do Cabo, Rio de Janeiro, Brazil., Iheringia Series Zoologie 95(1): 57-64
da Rocha, Rosana M.; Faria, Suzana B.; Moreno, Tatiane R. (2005) Ascidians from Bocas del Toro, Panama, Caribbean Journal of Science 41(3): 600-612
da Rocha, Rosana M.; Kremer, Laura P. (2005) Introduced ascidians in Paranagua Bay, Parana, southern Brazil., Revista Brasileira da Zoologia 22(4): 1170-1184
Dias, G. M.; Rocha, R. M.; Lotufo, T. M. C.; Kremer, L. P. (2013) Fifty years of ascidian biodiversity research in Sao Sebastiao, Brazil, Journal of the Marine Biological Association of the United Kingdom 93(1): 273-282
Dias, Gustavo Muniz; Delboni, Cynthia Grazielle Martins; Duarte, Luiz Francisco Lembo (2008) Effects of competition on sexual and clonal reproduction of a tunicate: the importance of competitor identity, Marine Ecology Progress Series 362: 149-156
Goodbody, Ivan; Webber, Mona (2003) <missing title>, 3 Environmental Foundation of Jamaica, Kingston. Pp. <missing location>
Granthom-Costa, Luciana Vieira; Werner Ferreira, Carlos Gustavo; Dias, Gustavo Muniz (2016) Biodiversity of ascidians in a heterogeneous bay from southeastern Brazil, Management of Biological Invasions 7: 5-12
Kott, P. (2005) Catalogue of Tunicata in Australian waters, Queensland Museum, Brisbane. Pp. 1-301
Lambert, Gretchen; Faulkes, Zen; Lambert, Charles C.; Scofield, Virginia L. (2005) Ascidians of South Padre Island, Texas, with a key to species., Texas Journal of Science 57(3): 251-262
Lord, Joshua P.; Calini, Jeremy M.; Whitlatch, Robert B. (2015) Influence of seawater temperature and shipping on the spread and establishment of marine fouling species, Marine Biology 162: 2481-2492
Marins, Flavia O.; Novaes, Roberto L. M.; Rocha, Rosana M.; Junquiera, Andrea O. R. (2010) Non indigenous ascidians in port and natural environments in a tropical Brazilian bay, Zoologia 27(2): 213-222
Monniot, C.; Monniot, F. (1985) [Littoral ascidians of Guadeloupe Island: IX. Characteristics of populations, ecology, relationships with the world fauna] (French), Tethys 11(3-4): 203-213
Monniot, Claude (1972) [Stolidobranch Ascidians of Bermuda[ (French), Bulletin du Museum National d'Histoire Naturelle. 4e Serie. Section A. Zoologie, Biologie et Ecologie Animales 43: 617-643
Monniot, Claude (2002) Stolidobranch ascidians from the tropical western Indian Ocean., Zoological Journal of the Linnean Society 135: 65-120
Monniot, Francoise; Monniot, Claude (1997) Ascidians collected in Tanzania, Journal of East African Natural History 86: 1-35
Quintanilla, Elena; Thomas Wilke; Ramırez-Portilla, Catalina; Sarmiento, Adriana; Sanchez, Juan A.2017 (2017) Taking a detour: invasion of an octocoral into the Tropical Eastern Pacific, Biological Invasions <missing volume>(17): 2583–2597
DOI 10.1007/s10530-017-1469-2
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/